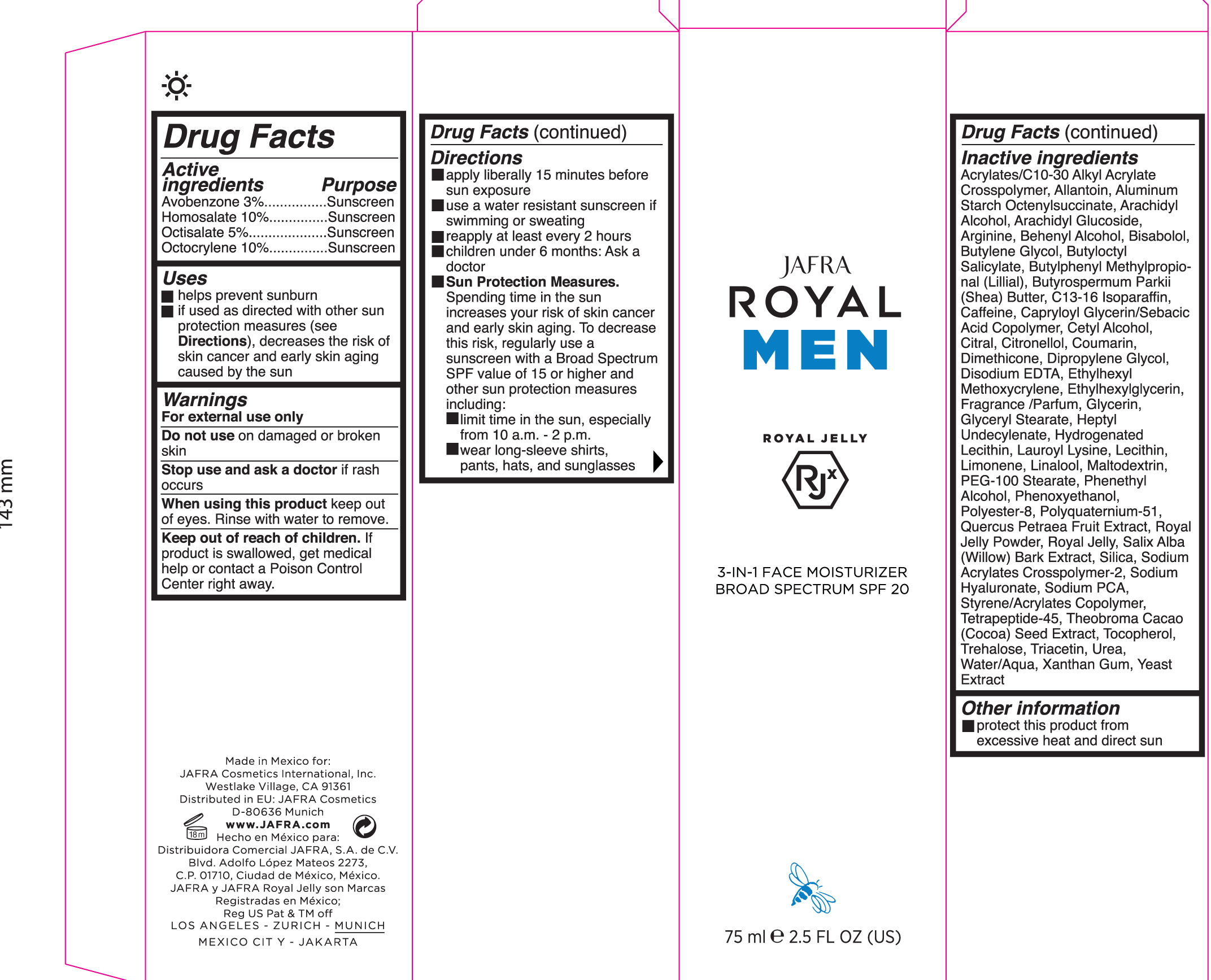
Active ingredient Purpose
Avobenzone 3% Sunscreen
Homosalate 10% Sunscreen
Octisalate 5% Sunscreen
Octocrylene 10% Sunscreen
Uses
Helps Prevent Sunburn
If used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children
If product is swallowed, get medical help or contact a poison Control Center right away
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- reapply at leastevery 2 hours.
- Children under 6 months of age: ask a doctor.
•
Sun Protection Measures Spending time in the sun increases your risk of
skin cancer and early skin aging. To decrease this risk, regularly use a
sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun
protection measures including:
- limit your time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Inactive ingredients: Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Allantoin, Aluminum Starch Octenylsuccinate, Arachidyl
Alcohol, Arachidyl Glucoside, Arginine, Behenyl Alcohol, Blsabolol, Butylene Glycol, Butyloctyl Salicylate, Butylphenyl Methylpropional (LIIIIal), Butyrospanmum Parkll (Shea) Butter, C13-16 Isoparaffin, Caffeine, Capryloyl Glycerin/Sabacic Acid Copolymer, Cetyl Alcohol, citral, citronellol, Coumarin, Dimethicone, Dipropylene Glycol, Disodium EDTA, Ethylhexyl Methoxycrylene, Ethylhexylglycerin, Fragrance/Parfum, Glycerin, Glyceryl Stearate, Heptyl Undecylenate, Hydrogenated Lecithin, Lauroyl Lysine, Lecithin, Limonene, Linalool, Maltodextrin, PEG-100 Stearate, Phenethyl Alcohol, Phenoxyethanol, Polyester-8, Polyquatemlum-51, Quercus Petraea Fruit Extract, Royal Jelly Powder, Royal Jelly, Salix Alba (Willow) Bark Extract, Silica, Sodium Acrylates Crosspolymer-2, Sodium Hyaluronate, Sodium PCA, Styrene/Acrylates Copolymer, Tetrapeptide-45, Theobroma Cacao (Cocoa) Seed Extract, Tocopherol, Trehalose, Triacetin, Urea, Water/Aqua, Xanthan Gum, Yeast Extract