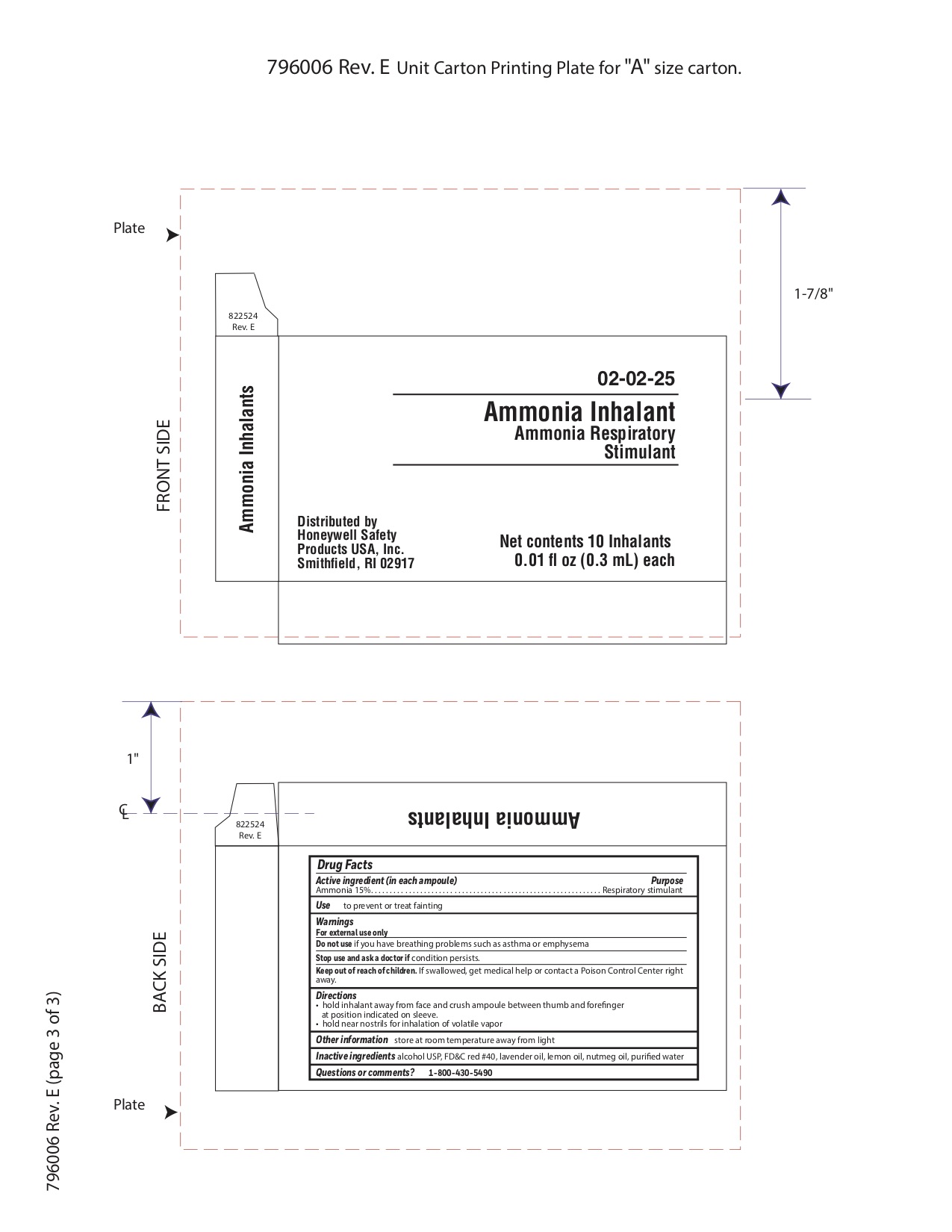
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
PVP
Warnings
For external use only
PVP
Directioons
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
PVP
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
4233
010998-4407 Kit Contents
1 AMMONIA INHALANTS ONTIC 10 PER
4 GAUZE BNDG ONTIC 4" X 6 YD
5 TRIANG BNDG ONTIC MUSLIN 1 PER
2 WIRE SPLINT ONTIC 1 PER
1 ADH TAPE ONTIC 1"X2-1/2YD 2P
8 BNDG COMP ONTIC 4"OFFSET 1PER
1 ADH BNDG ONTIC PL 1"X3" 16 PER
1 Cool Gel 1/8OZ 6PER
2 PVP WIPES ONTIC
1 NITRILE GLOVES ONTIC 2PR BAGGED
LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
1 STOCK LABEL 1 7/8" X 1/2"
1 KIT STL 24 UN WHITE 01
1 SCISSORS ANGLED 1EA IN BAG
1 LBL INSTR AVOX 10007143
4262
SF00000064 Kit Contents
2 AMMONIA INHALANTS 10 PER
2 TRIANGULAR BDG, NON-STERILE
2 GAUZE COMP, 18" X 36", 2 PER
2 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 PVP IODINE WIPES 10 PER
1 TWEEZER PLASTICS 4"
1 EMERGENCY SURVIVAL BLANKET
2 GAUZE CLEAN-WRAP BDGE N/S 2"
2 ELASTIC BANDAGE 3" X 4.5YD
1 SCISSOR BDGE 4" RED PLS HDL
1 BANDAGE COMP 2" W/TELFA PAD 4
1 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 24 UN WHITE 01
1 ADHS TAPE .5"X2.5YD 2
3 GAUZE PADS 3"X3" 4/BX