Approved by FDA under NADA # 128-409
1% Sterile Solution
A Parasiticide for the Treatment and Control of Internal and External Parasites of Cattle and Swine
Consult your veterinarian for assistance in the diagnosis, treatment and control of parasitism.
INTRODUCTION
IVOMEC® (ivermectin) is an injectable parasiticide for cattle and swine. One low-volume dose effectively treats and controls the following internal and external parasites that may impair the health of cattle and swine: gastrointestinal roundworms (including inhibited Ostertagia ostertagi in cattle), lungworms, grubs, sucking lice, and mange mites of cattle; and gastrointestinal roundworms, lungworms, lice, and mange mites of swine. Discovered and developed by scientists from Merck Research Laboratories, ivermectin is a novel chemical entity. Its convenience, broad-spectrum efficacy, and safety margin make IVOMEC Injection a unique product for parasite control of cattle and swine.
PRODUCT DESCRIPTION
Ivermectin is derived from the avermectins, a family of potent, broad-spectrum antiparasitic agents isolated from fermentation of Streptomyces avermitilis.
IVOMEC Injection is a clear, ready-to-use, sterile solution containing 1% ivermectin, 40% glycerol formal, and propylene glycol, q.s. ad 100%. IVOMEC Injection is formulated to deliver the recommended dose level of 200 mcg ivermectin/kilogram of body weight in cattle when given subcutaneously at the rate of 1 mL/110 lb (50 kg). In Swine, IVOMEC Injection is formulated to deliver the recommended dose level of 300 mcg ivermectin/kilogram body weight when given subcutaneously in the neck at the rate of 1 mL per 75 lb (33 kg).
MODE OF ACTION
Ivermectin is a member of the macrocyclic lactone class of endectocides which have a unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The margin of safety for compounds of this class is attributable to the fact that mammals do not have glutamate-gated chloride channels, the macrocyclic lactones have a low affinity for other mammalian ligandgated chloride channels and they do not readily cross the blood-brain barrier.
INDICATIONS
Cattle: IVOMEC Injection is indicated for the effective treatment and control of the following harmful species of gastrointestinal roundworms, lungworms, grubs, sucking lice, and mange mites in cattle:
Gastrointestinal Roundworms (adults and fourth-stage larvae): Ostertagia ostertagi (including inhibited O. ostertagi), O. lyrata, Haemonchus placei, Trichostrongylus axei, T. colubriformis, Cooperia oncophora, C. punctata, C. pectinate, Oesophagostomum radiatum, Bunostomum phlebotomum, Nematodirus helvetianus (adults only), N. spathiger (adults only)
Lungworms (adults and fourth-stage larvae): Dictyocaulus viviparus
Cattle Grubs (parasitic stages): Hypoderma bovis, H. lineatum
Sucking Lice:Linognathus vituli, Haematopinus eurysternus, Solenopotes capillatus
Mites (scabies): Psoroptes ovis (syn. P. communis var. bovis), Sarcoptes scabiei var. bovis
Persistent Activity
IVOMEC Injection has been proved to effectively control infections and to protect cattle from reinfection with Dictyocaulus viviparus and Oesophagostomum radiatum for 28 days after treatment; Ostertagia ostertagi, Trichostrongylus axei and Cooperia punctata for 21 days after treatment; Haemonchus placei and Cooperia oncophora for 14 days after treatment.
Swine: IVOMEC Injection is indicated for the effective treatment and control of the following harmful species of gastrointestinal roundworms, lungworms, lice, and mange mites in swine:
Gastrointestinal Roundworms: Large roundworm, Ascaris suum (adults and fourth-stage larvae), Red stomach worm, Hyostrongylus rubidus (adults and fourth-stage larvae), Nodular worm, Oesophagostomum spp. (adults and fourth-stage larvae), Threadworm, Strongyloides ransomi (adults)
Somatic Roundworm Larvae: Threadworm, Strongyloides ransomi (somatic larvae)
Sows must be treated at least seven days before farrowing to prevent infection in piglets.
Lungworms:Metastrongylus spp. (adults)
Lice:Haematopinus suis
Mange Mites:Sarcoptes scabiei var. suis
DOSAGE
Cattle: IVOMEC Injection should be given only by subcutaneous injection under the loose skin in front of or behind the shoulder at the recommended dose level of 200 mcg of ivermectin per kilogram of body weight. Each mL of IVOMEC contains 10 mg of ivermectin, sufficient to treat 110 lb (50 kg) of body weight (maximum 10 mL per injection site).
| Body Weight (lb) | Dose Volume (mL) |
|---|---|
|
220 |
2 |
|
330 |
3 |
|
440 |
4 |
|
550 |
5 |
|
660 |
6 |
|
770 |
7 |
|
880 |
8 |
|
990 |
9 |
|
1100 |
10 |
Swine: IVOMEC Injection should be given only by subcutaneous injection in the neck of swine at the recommended dose level of 300 mcg of ivermectin per kilogram (2.2 lb) of body weight. Each mL of IVOMEC contains 10 mg of ivermectin, sufficient to treat 75 lb of body weight.
| Body Weight (lb) | Dose Volume (mL) | |
|---|---|---|
|
Growing Pigs |
19 |
1/4 |
|
38 |
1/2 |
|
|
75 |
1 |
|
|
150 |
2 |
|
|
Breeding Animals |
225 |
3 |
|
(Sows, Gilts, and Boars) |
300 |
4 |
|
375 |
5 |
|
|
450 |
6 |
Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.
ADMINISTRATION
Any single-dose syringe or standard automatic syringe equipment may be used with the 50 mL bottle. Use the 50 mL bottle within 6 months of first puncture and puncture a maximum of 12 times. If more than 12 punctures are anticipated, the use of multi-dosing equipment is recommended. When using a draw-off spike or needle with bore diameter larger than 16-gauge, discard any product remaining in the bottle immediately after use. When using the 200 mL, 500 mL or 1000 mL pack size, use only automatic syringe equipment. Discard any product remaining in the pack immediately after use.
Use sterile equipment and sanitize the injection site by applying a suitable disinfectant. Clean, properly disinfected needles should be used to reduce the potential for injection site infections. The rubber stopper should also be disinfected with alcohol to prevent contamination of the contents. No special handling or protective clothing is necessary.
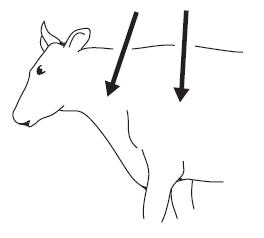
Cattle:IVOMEC (ivermectin) Injection is to be given subcutaneously only, to reduce risk of potentially fatal clostridial infection of the injection site. Animals should be appropriately restrained to achieve the proper route of administration. Use of a 16-gauge, 1/2 to 3/4" needle is suggested. Inject under the loose skin in front of or behind the shoulder (see illustration).
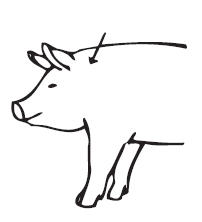
Swine: IVOMEC Injection is to be given subcutaneously in the neck. Animals should be appropriately restrained to achieve the proper route of administration. Use of a 16- or 18-gauge needle is suggested for sows and boars, while an 18- or 20-gauge needle may be appropriate for young animals. Inject under the skin, immediately behind the ear (see illustration). Mild and transient pain reactions may be seen in some swine following subcutaneous administration.
Recommended Treatment Program
Swine: At the time of initiating any parasite control program, it is important to treat all breeding animals in the herd. After the initial treatment, use IVOMEC Injection regularly as follows:
BREEDING ANIMALS
Sows: Treat prior to farrowing, preferably 7–14 days before, to minimize infection of piglets.
Gilts: Treat 7–14 days prior to breeding.
Treat 7–14 days prior to farrowing.
Boars: Frequency and need for treatments are dependent upon exposure. Treat at least two times a year.
FEEDER PIGS
(Weaners/Growers/Finishers)
All weaner/feeder pigs should be treated before placement in clean quarters.
Pigs exposed to contaminated soil or pasture may need retreatment if reinfection occurs.
NOTE:
- (1)
- IVOMEC Injection has a persistent drug level sufficient to control mite infestations throughout the egg to adult life cycle. However, since the ivermectin effect is not immediate, care must be taken to prevent reinfestation from exposure to untreated animals or contaminated facilities. Generally, pigs should not be moved to clean quarters or exposed to uninfested pigs for approximately one week after treatment. Sows should be treated at least one week before farrowing to minimize transfer of mites to newborn baby pigs.
- (2)
- Louse eggs are unaffected by IVOMEC Injection and may require up to three weeks to hatch. Louse infestations developing from hatching eggs may require retreatment.
- (3)
- Consult a veterinarian for aid in the diagnosis and control of internal and external parasites of swine.
Special Minor Use
Reindeer: For the treatment and control of warbles (Oedemagena tarandi) in reindeer, inject 200 micrograms ivermectin per kilogram of body weight, subcutaneously. Follow use directions for cattle as described under ADMINISTRATION.
American Bison: For the treatment and control of grubs (Hypoderma bovis) in American bison, inject 200 micrograms ivermectin per kilogram of body weight, subcutaneously. Follow use directions for cattle as described under ADMINISTRATION.
WARNING
NOT FOR USE IN HUMANS.
Keep this and all drugs out of the reach of children.
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report suspected adverse drug events, for technical assistance, or to obtain a copy of the SDS, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or at www.fda.gov/reportanimalae.
RESIDUE WARNING
Do not treat cattle within 35 days of slaughter. Because a withdrawal time in milk has not been established, do not use in female dairy cattle of breeding age. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal. Do not treat swine within 18 days of slaughter.
PRECAUTIONS
Transitory discomfort has been observed in some cattle following subcutaneous administration. A low incidence of soft tissue swelling at the injection site has been observed. These reactions have disappeared without treatment. For cattle, divide doses greater than 10 mL between two injection sites to reduce occasional discomfort or site reaction. Use sterile equipment and sanitize the injection site by applying a suitable disinfectant. Clean, properly disinfected needles should be used to reduce the potential for injection site infections.
Observe cattle for injection site reactions. Reactions may be due to clostridial infection and should be aggressively treated with appropriate antibiotics. If injection site infections are suspected, consult your veterinarian.
This product is not for intravenous or intramuscular use.
IVOMEC Injection for Cattle and Swine has been developed specifically for use in cattle, swine, reindeer, and American bison only. This product should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
Restricted Drug (California) - use only as directed.
When to Treat Cattle with Grubs
IVOMEC effectively controls all stages of cattle grubs. However, proper timing of treatment is important. For most effective results, cattle should be treated as soon as possible after the end of the heel fly (warble fly) season. Destruction of Hypoderma larvae (cattle grubs) at the period when these grubs are in vital areas may cause undesirable host-parasite reactions including the possibility of fatalities. Killing Hypoderma lineatum when it is in the tissue surrounding the esophagus (gullet) may cause salivation and bloat; killing H. bovis when it is in the vertebral canal may cause staggering or paralysis. These reactions are not specific to treatment with IVOMEC, but can occur with any successful treatment of grubs. Cattle should be treated either before or after these stages of grub development. Consult your veterinarian concerning the proper time for treatment. Cattle treated with IVOMEC after the end of the heel fly season may be retreated with IVOMEC during the winter for internal parasites, mange mites, or sucking lice without danger of grub-related reactions. A planned parasite control program is recommended.
OTHER WARNINGS
Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers.
Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance.
Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd, prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method).
A decrease in a drug's effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring.
Environmental Safety
Studies indicate that when ivermectin comes in contact with soil, it readily and tightly binds to the soil and becomes inactive over time. Free ivermectin may adversely affect fish and certain aquatic organisms. Do not permit water runoff from feed lots to enter lakes, streams or ponds. Do not contaminate water by direct application or by improper disposal of drug containers. Dispose of containers in an approved landfill or by incineration.
As with other avermectins, ivermectin is excreted in the dung of treated animals and can inhibit the reproduction and growth of pest and beneficial insects that use dung as a source of food and for reproduction. The magnitude and duration of such effects are species and life-cycle specific. When used according to label directions, the product is not expected to have an adverse impact on populations of dung-dependent insects.
STORAGE CONDITIONS
Store at or below 25°C (77°F) with excursions permitted up to 30°C (86°F). Protect product from light.
HOW SUPPLIED
IVOMEC Injection for Cattle and Swine is available in four ready-to-use pack sizes:
The 50 mL pack is a multiple-dose, rubber-capped bottle. Each bottle contains sufficient solution to treat 10 head of 550 lb (250 kg) cattle or 100 head of 38 lb (17.3 kg) swine.
The 200 mL pack is a soft, collapsible pack designed for use with automatic syringe equipment. Each pack contains sufficient solution to treat 40 head of 550 lb (250 kg) cattle or 400 head of 38 lb (17.3 kg) swine.
The 500 mL pack is a soft, collapsible pack designed for use with automatic syringe equipment. Each pack contains sufficient solution to treat 100 head of 550 lb (250 kg) cattle or 1000 head of 38 lb (17.3 kg) swine.
The 1000 mL is a soft, collapsible pack designed for use with automatic syringe equipment. Each pack contains sufficient solution to treat 200 head of 550 lb (250 kg) cattle or 2000 head of 38 lb (17.3 kg) swine.