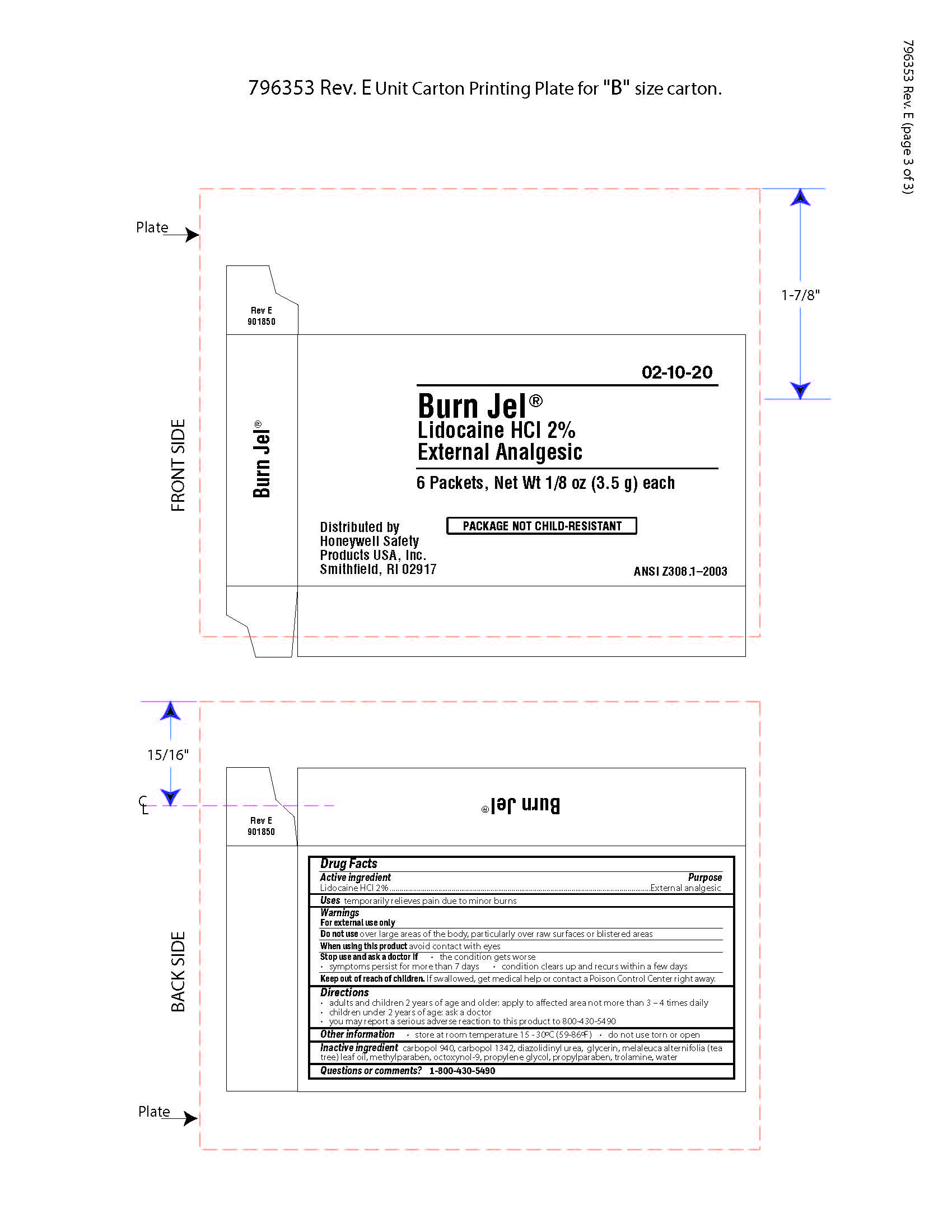
Burn Jel
Warnings
For external use only
Burn JEl
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
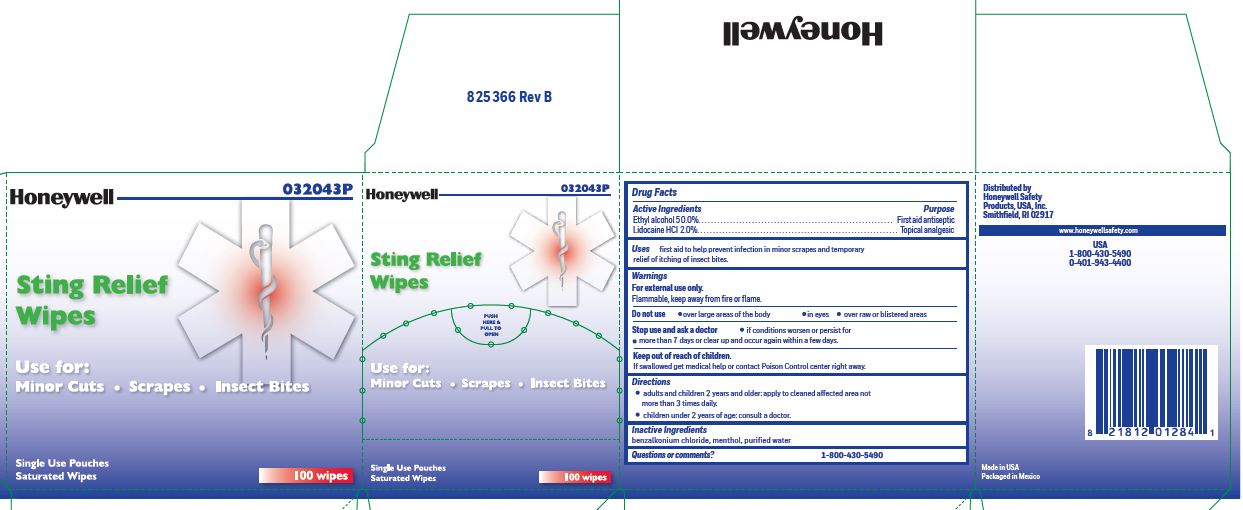
Sting Relief
Uses
prevent infection in minor scrapes, and temporary relief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable,
keep away from open fire or flame
Sting Relief
Directions
- adults and children 2 years and older:
- apply to cleaned affected area not more than 3 times daily.
- children under 2 years of age: consult a doctor.
PVP
Warnings
For external use only
PVP
Directioons
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
PVP
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
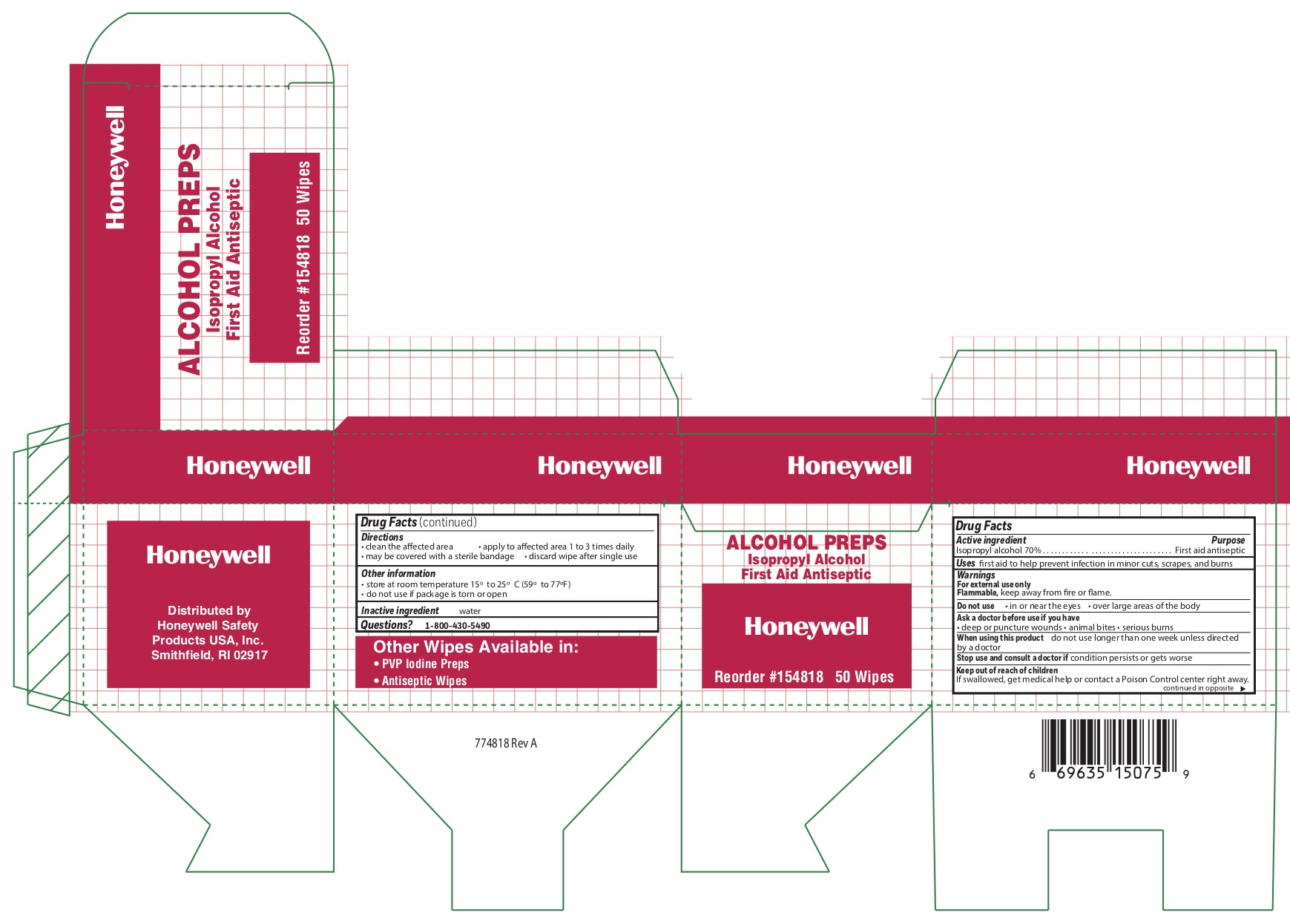
Alcohol
Directioons
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily
- discard wipe after single use
Alcohol
Other information
- store at room temperature 15 o to 25 o C (59 o to 77 oF)
- do not use if packet is torn or opened
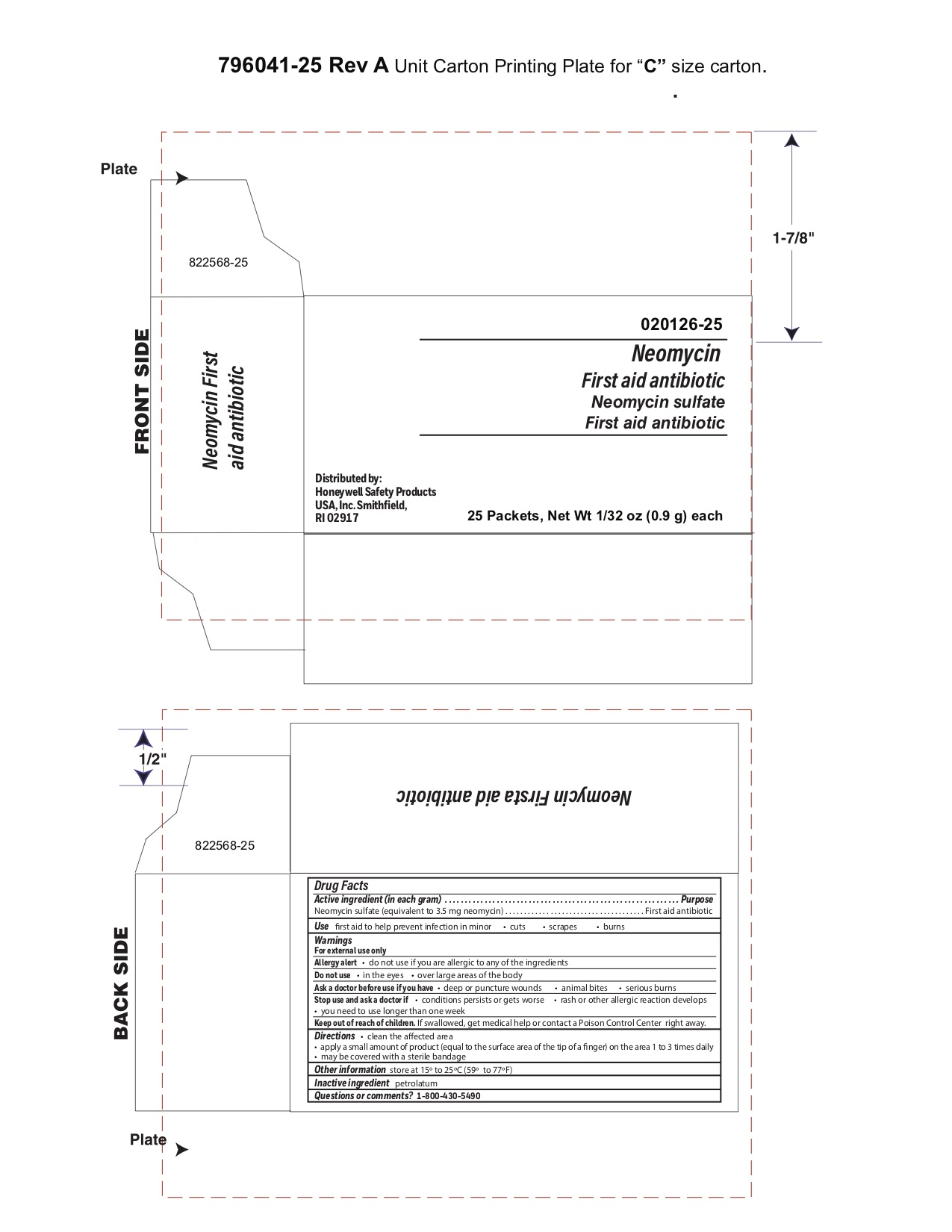
Neomycin
Warnings
For external use only
Neomycin
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only
- Obtain immediate medical treatment for all open wounds in or near eyes.
- To avoid contamination, do not touch tip of container to any surface.
- Do not reuse.
- Once opened, discard
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
4220
019643-4367 Kit Contents
1 KNUCKLE BAND 8 PER
1 NEOMYCIN ANTIBIOTIC 10 PER
1 GAUZE BANDAGE, 4" X 6 YD
2 TRIANGULAR BDG, NON-STERILE
1 GAUZE PADS, 3" X 3", 4 PER
1 ADHESIVE TPE 1"X2-1/2 YD 2 PER
2 INSTANT COLD PACK 4" X 6"
2 BANDAGE COMP, 2" OFFSET, 4 PER
2 BANDAGE COMP, 4" OFFSET, 1 PER
2 ADH BAND, EXTRA LARGE, 6 PER
2 1 OZ EYE WASH W/PADS & STRIPS
1 BURN JEL 1/8 OZ, 6 PER
5 WATER JEL DRESSING,2" X 6"
1 PVP IODINE WIPES 10 PER
2 NITRILE GLOVES 2PR BBP
2 ADH BDG, CLOTH, 1"X3", 16 PER
1 FIRST AID GUIDE ASHI
2 ABD COMBINE PAD 5" X 9"
1 MICROSHIELD W/VNL GLV/ALCL
1 SCISSOR BDGE 4" RED PLS HDL
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 STOCK LABEL 1 7/8" X 1/2"
1 LBL CONTS 8"X8",CUSTOM ID B
1 KIT 36 UNIT PLASTIC
1 STING Relief WIPES 10