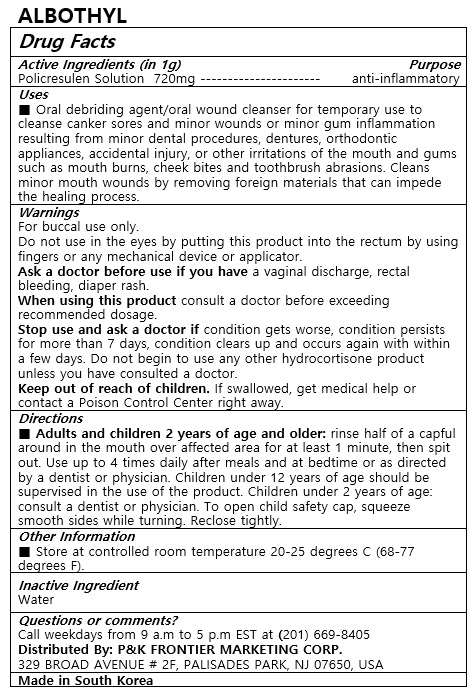
Oral debriding agent/oral wound cleanser for temporary use to cleanse canker sores and minor wounds or minor gum inflammation resulting from minor dental procedures, dentures, orthodontic appliances, accidental injury, or other irritations of the mouth and gums such as mouth burns, cheek bites and toothbrush abrasions. Cleans minor mouth wounds by removing foreign materials that can impede the healing process.
Adults and children 2 years of age and older: rinse half of a capful around in the mouth over affected area for at least 1 minute, then spit out. Use up to 4 times daily after meals and at bedtime or as directed by a dentist or physician. Children under 12 years of age should be supervised in the use of the product. Children under 2 years of age: consult a dentist or physician. To open child safety cap, squeeze smooth sides while turning. Reclose tightly.
For buccal use only.
Do not use in the eyes by putting this product into the rectum by using fingers or any mechanical device or applicator.
Ask a doctor before use if you have a vaginal discharge, rectal bleeding, diaper rash.
When using this product consult a doctor before exceeding recommended dosage.
Stop use and ask a doctor if condition gets worse, condition persists for more than 7 days, condition clears up and occurs again with within a few days. Do not begin to use any other hydrocortisone product unless you have consulted a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.