DESCRIPTION:
Bioaches Patch contains capsaicin and menthol in a localized dermal delivery system. The capsaicin in Bioaches Patch is a synthetic equivalent of the naturally occurring compound found in chili peppers. Capsaicin is soluble in alcohol, acetone, and ethyl acetate and very slightly soluble in water. The menthol is slightly soluble in water.
Bioaches Patch is a single-use patch stored in a foil pouch. Each Bioaches Patch is 7.6 cm x 12.7 cm (97 cm²) and consists of a polyester backing film coated with a drug-containing silicone adhesive mixture, and covered with a removable polyester release liner.
The empirical formula for capsaicin is C H NO , with a molecular weight of 305.42. The chemical compound capsaicin [(E)-8-methyl-N-vanillyl-6-nonenamide] is an activating ligand for transient receptor potential vanilloid 1 receptor (TRPV1) and it has the following structure:
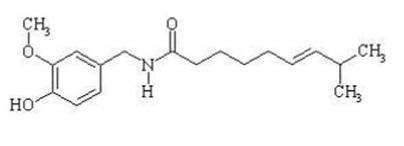
The empirical formula for menthol is C H O, with a molecular weight of 156.27. The chemical compound menthol [(1R,2S,5R)-2-isopropyl-5-methylcyclohexanol ] is an activating ligand for transient receptor potential cation channel subfamily M member 8 (TRPM8). It has the following structure:
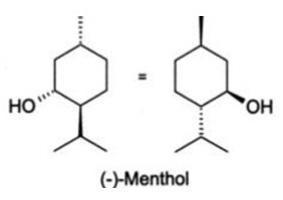
Each Bioaches Patch contains 0.0375% capsaicin (0.0375 grams of capsaicin per patch) and 5.00% menthol (5 grams of menthol per patch).
INDICATIONS AND USAGE
Bioaches Patch is indicated for the temporary relief of minor aches and muscle pains associated with arthritis, simple backache, strains, muscle soreness and stiffness.
Bioaches Patch is an external analgesic/counterirritant.
CONTRAINDICATIONS
Bioaches Patch is contraindicated for those patients with a history of hypersensitivity to any of the components of the preparation.
WARNINGS
• For external use only.
• Use only as directed.
• Avoid contact with eyes and mucous membranes.
• Do not cover with bandage.
• Do not use on wounds or damaged skin.
• Consult physician for children under 12.
• Do not use if you are allergic to Menthol.
• Stop use and ask a doctor if conditions worsen, symptoms persist for more than 7 days or clear up and occur again within a few days or rash, itching or excessive skin irritation occurs.
• KEEP OUT OF REACH OF CHILDREN
DOSAGE AND ADMINISTRATION
Bioaches patch contains 0.0375% capsaicin and 5.00% menthol
Instructions for Use
Clean and dry affected area
Tear open pouch. Unseal bag and remove patch
Remove protective film and apply directly to area of pain
Apply to affected area not more than 3 times daily
Wash hands with soap after applying patch
Reseal pouch containing unused patches
Directions
| Adults and children 12 years and over |
| |
| ||
| Children under 12 years |
|
Inactive Components: Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Diazolidinyl Urea, EDTA Disodium Salt, Glycerin, lodopropynyl Butyl carbamate, Methylparaben, Polysorbate 80, Propylparaben. Sodium Polyacrylate and Water.
