Eyesaline
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyesaline
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyesaline
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyesaline
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
Povidone Iodine Swab
Active ingredient
Povidone-iodine solution USP, 10% (equivalent to 1% titratable iodine)
Povidone Iodine Swab
Uses
- first aid to help prevent the risk of infection in minor cuts, scrapes, and burns
Povidone Iodine Swab
Directions
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
Povidone Iodine Swab
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
Povidone Iodine Swab
Inactive ingredients
citric acid, disodium phosphate,nonoxynol-9, sodium hydroxide, water
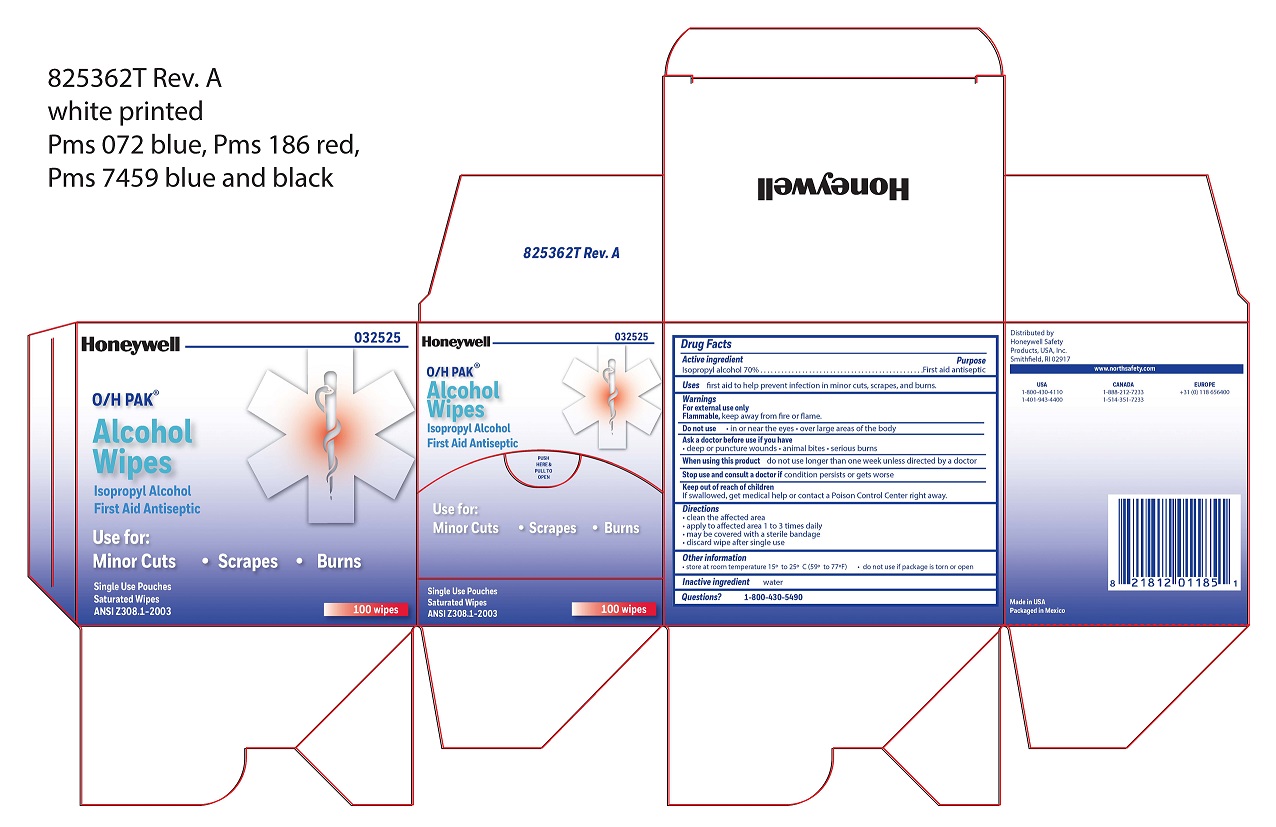
Alcohol Wipe
Directions
- clean the affected area
- apply wipe to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard wipe after single use
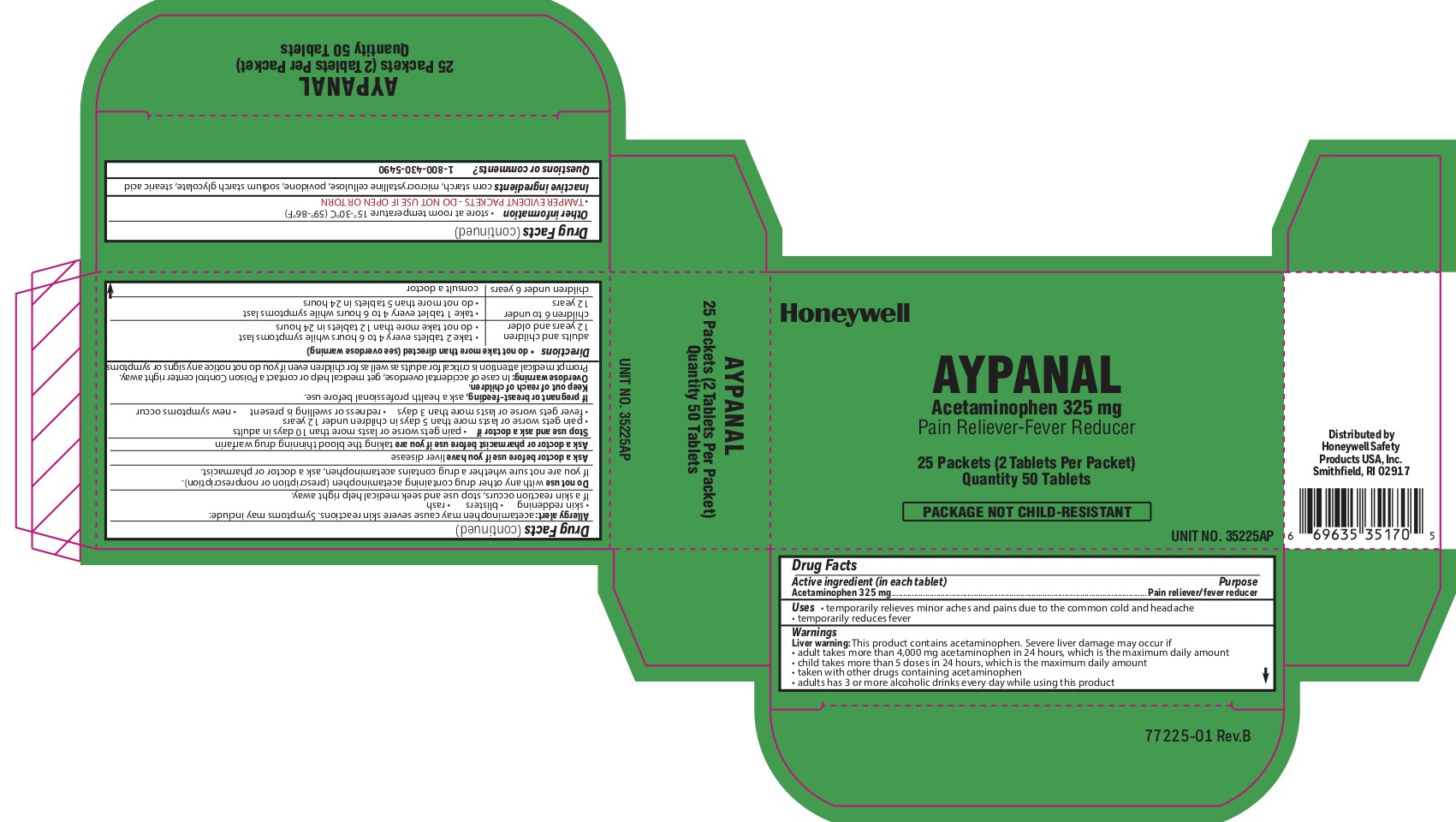
Aypanaly
Uses
- temporarily relieves minor aches and pains due to the common cold and headache - temporarily reduces fever
Aypanal
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product:
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
- If a skin rash occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Aypanal
Directions
do not take more than directed (see overdose warning)
adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than 12 tablets in 24 hours
children 6 to under 12 years of age
- take 1 tablet every 4-6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
children under 6 years consult a doctor
Aypanal
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
Aypanal
Inactive ingredients
corn starch, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
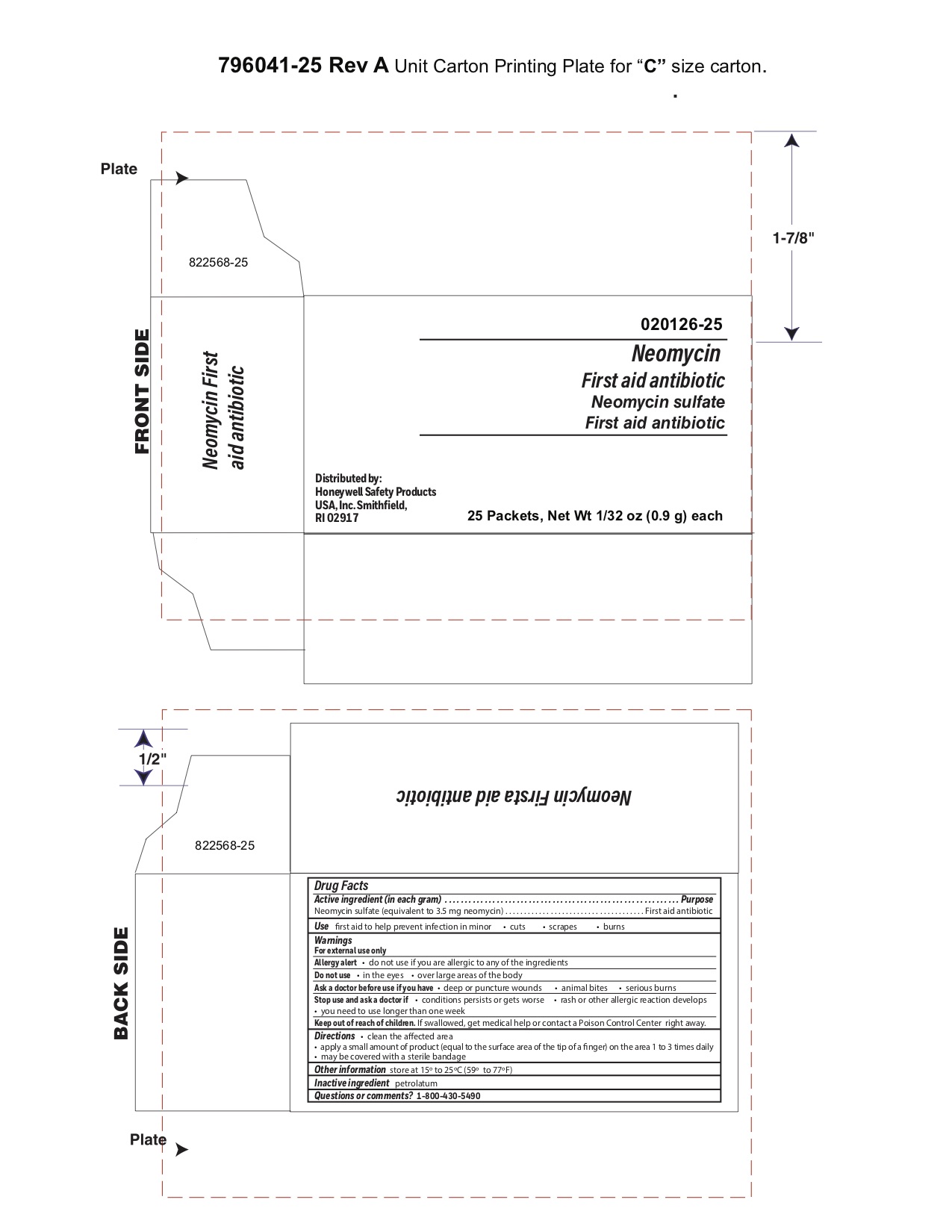
Neomycin
Warnings
For external use only
Stop use and ask a doctor if
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Neomycin
Direction
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Antiseptic Spray
Directions
- clean the affected area
- spray a small amount of this product on the area 1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
Antiseptic Spray
Inactive ingredients
diazolidinyl urea, edetate disodium, glycerin, hypromellose, methylparaben, octoxynol 9, propylene glycol, propylparaben, trolamine, water
Burn Spray
Uses
for the temporary relief of pain and itching and helps protect against infection in:
- minor cuts and scrapes
- burns
- sunburn
- insect bites
- minor skin irritations
Burn Spray
Warnings
For external use only
Flammable
- keep away from fire or flame
- contents under pressure
- do not puncture or incinerate container
- do not expose to temperatures above 120 0 F
Do not use
- in or near the eyes or other mucous membranes
- in case of serious burns
- in case of deep or puncture wounds
- for prolonged period of time
- on large portion of the body
Burn Spray
Directions
- clean the affected area
- shake can well before using
- hold 4 - 6 inches from surface and spray area until wet
- may be covered with a sterile bandage, if bandaged let dry first
- for adult institutional use only
- not intended for use on children
Burn Spray
Other information
- avoid inhaling
- use only as directed
- intentional misuse by deliberately concentrating or inhaling the contents may be harmful or fatal
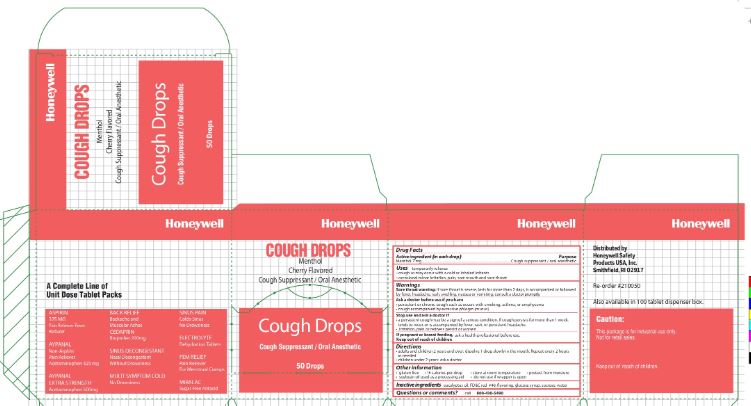
Cough Drop
Uses
temporarily relieves:
- cough as may occur with a cold or inhaled irritants
- occasional minor irritation, pain, sore mouth and sore throat
Cough Drop
Warnings
Sore throat warning: if sore throat is severe, lasts for more than 2 days, is accompanied or followed by a fever, headache, rash, swelling, nausea or vomiting, consult a doctor promptly
Ask a doctor before use if you have:
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough accompanied by excessive phlegm (mucus)
Cough Drop
Directions
- adults and children 2 years of age and over
- dissolve 1 drop slowly in the mouth. Repeat every 2 hours as needed
- children under 2 years of age: ask a doctor
Cough Drop
Other information
- gluten free
- 15 calories per cough drop
- store at room temperature
- protect from moisture
- soybean oil used as a processing aid
- do not use if wrapper is open
Cough Drop
Inactive ingredients
eucalyptus oil, FD&C red #40, flavoring, glucose syrup, sucrose, water
4210
019722-0011L Kit Contents
2 1X3 PLASTIC 100/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 SWIFT KNUCKLE 40/BX
2 3/4 X 3 WOVEN 100/BOX
3 NEOMYCIN ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
3 ALCOHOL PREP PADS 10P
4 PVP IODINE WIPES 10 PER
2 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 ADH BAND PLSTC EX-LG 25 PER
1 O/H PUMP ANTISEPTIC 2 OZ ID F
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 FIRST AID GUIDE ASHI
1 TAPE ADHESIVE 1"X 5 YD PLSTC
10 GAUZE CLEAN-WRAP BDGE N/S 2"
10 GAUZE CLEAN-WRAP BDGE N/S 3"
1 BLOODSTOPPER
3 NON-ADHERENT PADS 2"X3" 10'S
1 GZE PADS STERILE 2"X 2" 10'S
1 GZE PADS STERILE 3"X 3" 25'S
1 ELASTIC BANDAGE 3" X 4.5YD
1 CPR FILTERSHIELD 77-100
1 AYPANAL NON-ASP IND 2/ENV 250
1 CHERRY COUGH DROPS 50
2 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LABEL NORTH CONTENTS 8X8 ID B
5 PR LRG NITRILE GLVES ZIP BAG
1 KIT STL DELUXE FA CABINET
1 POCKET FA CABINET LARGE
1 SHELF LG FA CABINET
1 LBL CONTENTS ANSI Z308.1-2009 REV B
1 LBL CAB CVR FA LOGO NORTH ID B
8 CORNER STYROFOAM 3X3X3
2 TRI BNDG NON WOVEN 40"X40"X56"
4 COLD PACK UNIT 4"X6" BULK
4257
Z019721-0100L
2 1X3 PLASTIC 100/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 SWIFT KNUCKLE 40/BX
1 3/4 X 3 WOVEN 100/BOX
2 NEOMYCIN ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
3 ALCOHOL PREP PADS 10P
4 PVP IODINE WIPES 10 PER
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 ADH BAND PLSTC EX-LG 25 PER
1 O/H PUMP ANTISEPTIC 2 OZ ID F
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 FIRST AID GUIDE ASHI
1 TAPE ADHESIVE 1"X 5 YD PLSTC
5 GAUZE CLEAN-WRAP BDGE N/S 2"
5 GAUZE CLEAN-WRAP BDGE N/S 3"
1 BLOODSTOPPER
3 NON-ADHERENT PADS 2"X3" 10'S
1 GZE PADS STERILE 2"X 2" 10'S
1 GZE PADS STERILE 3"X 3" 25'S
1 ELASTIC BANDAGE 3" X 4.5YD
1 CPR FILTERSHIELD 77-100
1 AYPANAL NON-ASP IND 2/ENV 100
1 CHERRY COUGH DROPS 50
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
5 PR LRG NITRILE GLVES ZIP BAG
1 KIT STL LARGE FA CABINET
1 LBL CONTENTS ANSI Z308.1-2009 REV B
1 LBL CAB CVR FA LOGO NORTH ID B
2 TRI BNDG NON WOVEN 40"X40"X56"
3 COLD PACK UNIT 4"X6" BULK
4204 Kit Contnets
019721-0010L
2 1X3 PLASTIC 100/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 SWIFT KNUCKLE 40/BX
1 3/4 X 3 WOVEN 100/BOX
2 NEOMYCIN ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
3 ALCOHOL PREP PADS 10P
4 PVP IODINE WIPES 10 PER
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 ADH BAND PLSTC EX-LG 25 PER
1 O/H PUMP ANTISEPTIC 2 OZ ID F
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 FIRST AID GUIDE ASHI
1 TAPE ADHESIVE 1"X 5 YD PLSTC
5 GAUZE CLEAN-WRAP BDGE N/S 2"
5 GAUZE CLEAN-WRAP BDGE N/S 3"
1 BLOODSTOPPER
3 NON-ADHERENT PADS 2"X3" 10'S
1 GZE PADS STERILE 2"X 2" 10'S
1 GZE PADS STERILE 3"X 3" 25'S
1 ELASTIC BANDAGE 3" X 4.5YD
1 CPR FILTERSHIELD 77-100
1 AYPANAL NON-ASP IND 2/ENV 100
1 CHERRY COUGH DROPS 50
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LABEL NORTH CONTENTS 8X8 ID B
5 PR LRG NITRILE GLVES ZIP BAG
KIT STL LARGE FA CABINET
1 LBL CONTENTS ANSI Z308.1-2009 REV B
1 LBL CAB CVR FA LOGO NORTH ID B
1 BAG ZIPPER POLY 6 X 6 2 MIL
2 TRI BNDG NON WOVEN 40"X40"X56"
3 COLD PACK UNIT 4"X6" BULK