Active ingredients (in each capsule)
Acetaminophen 325 mg
Dextromethorphan HBr 10 mg
Phenylephrine HCl 5 mg
Uses
temporarily relieves common cold/flu symptoms:
- cough due to minor throat & bronchial irritation
- nasal congestion
- sore throat
- headache
- minor aches/pains
- fever
Alcohol warning: If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take acetaminophen or other pain relievers/fever reducers. Acetaminophen may cause liver damage.
Sore throat warning: If sore throat is severe, persists for more than two days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do Not Use
- with other medicines containing acetaminophen
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- diabetes
- high blood pressure
- trouble urinating due to enlarged prostate gland
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough as occurs with smoking, asthma, or emphysema
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- symptoms get worse or last more than 5 days (children) or 7 days (adults)
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back, or occurs with rash or headache that lasts.
These could be signs of a serious condition.
Directions
- take only as recommended - see OVERDOSE warning
- do not exceed 6 doses per 24 hours
| Adults and children 12 years of age and older | 2 LiquiCaps with water every 4 hours |
| Children under 12 years of age | ask doctor |
When using other DayQuil or NyQuil products, carefully read each label to insure correct dosing
Inactive ingredients
polyethylene glycol 400, propylene glycol, povidone k30, fd&c red no. 40, fd&c yellow no. 6, titanium dioxide, gelatin, glycerin, sorbitol, water
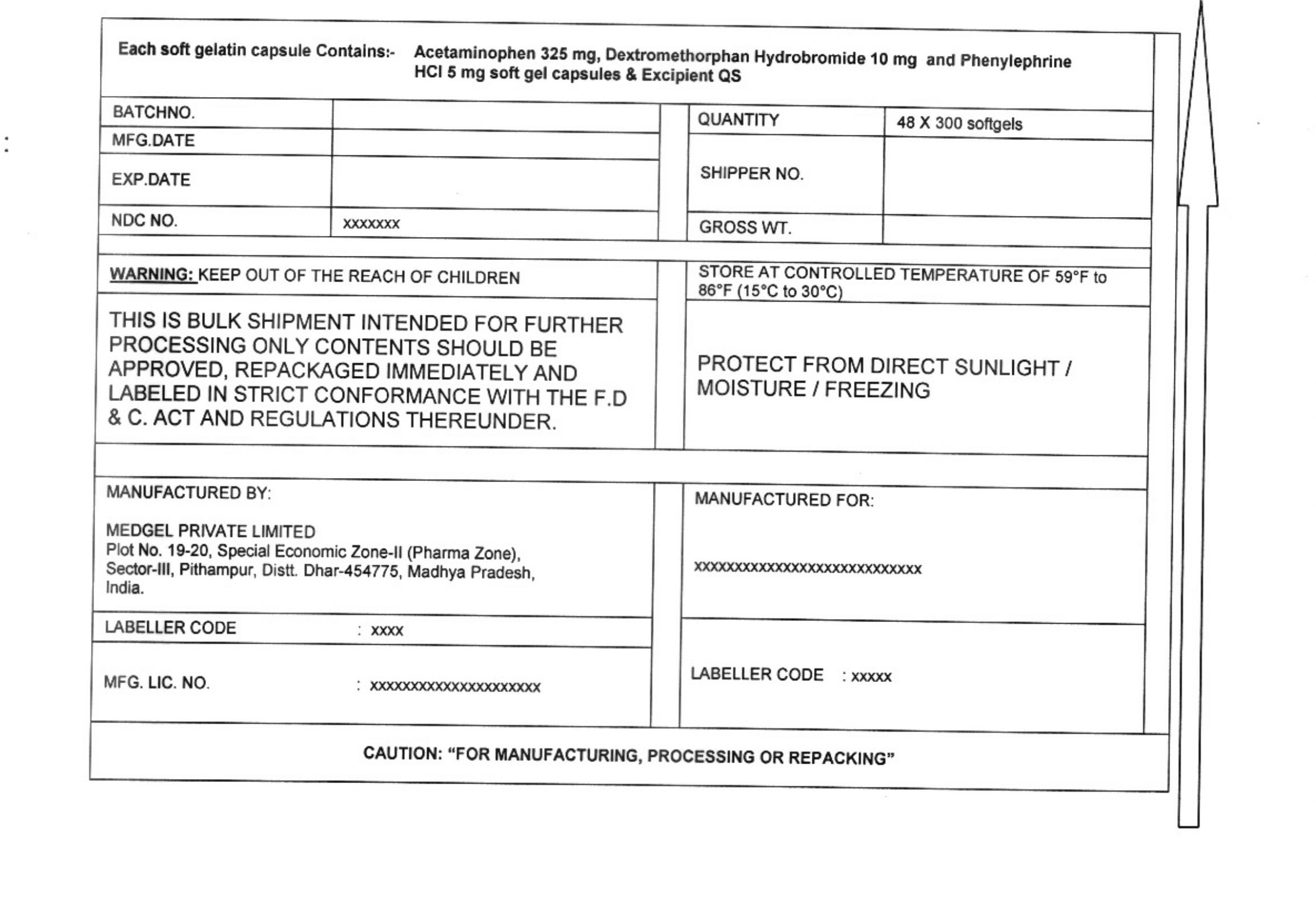
PRINCIPAL DISPLAY PANEL - Shipping Label
Acetaminophen, Dextromethorphan HBr Phenylephrine HCL capsules
Each Softgel Contains:
(Acetaminophen USP 325 mg, Dextromethorphan Hydrobromide USP 10 mg,
Phenylephrine Hydrochloride USP 5mg)
LOT NO:
DRUM NO:
MFG DATE:
QUANTITY:
NDC NO: 55629-012-
EXP DATE:
WARNING:
KEEP OUT OF REACH OF CHILDREN
STORE CONTROLLED ROOM TEMPERATURE OF 59° - 86°F (15° - 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZING
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT CONFORMANCE WITH
THE F.D & C.ACT AND REGULATIONS THEREUNDER.