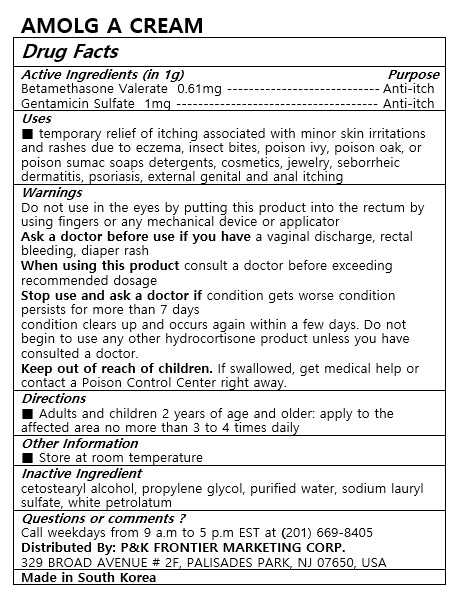
temporary relief of itching associated with minor skin irritations and rashes due to eczema, insect bites, poison ivy, poison oak, or poison sumac soaps detergents, cosmetics, jewelry, seborrheic dermatitis, psoriasis, external genital and anal itching
Adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
Do not use in the eyes by putting this product into the rectum by using fingers or any mechanical device or applicator
Ask a doctor before use if you have a vaginal discharge rectal bleeding
diaper rash
When using this product consult a doctor before exceeding recommended dosage
Stop use and ask a doctor if condition gets worse condition persists for more than 7 days
condition clears up and occurs again within a few days. Do not begin to use any other hydrocortisone product unless you have consulted a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.