AUSTRALIAN GOLD DEFENSE ZONE ANTI-DANDRUFF LEAVE-IN TREATMENT- pyrithione zinc lotion
Prime Enterprises, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient:
Pyrithione Zinc 0.22%
Indications:
- Controls the symptoms of dandruff.
- Helps prevent recurrence of scalp itching and flaking associated with dandruff.
Warning:
When using this product
- Avoid contact with the eyes. If contact occurs, rinse eyes thorougly with water.
Stop use and ask a doctor
- If condition worsens or does not improve after regular use of this product as directed.
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away
Directions:
- Shake well.
- Use after shampooing and towel drying hair. Part hair section by section. Apply directly onto the scalp and spread using fingertips. Gently massage into scalp. Leave in.
- Apply to affected areas one to four times daily or as directed by a doctor
- Children under 6 months of age: ask a doctor.
Inactive Ingrediets:
Caprylyl Glycol, Ceteareth-20, Cetearyl Alcohol, Cetrimonium Bromide, Cetyl Alcohol, Chlorphenesin, Citric Acid, Disodium EDTA, Elaeis Guineensis (Palm) Oil, Fragrance, Glycerin, Phenoxyethanol, Sodium Hydroxide, Stearyl Dihydroxypropyldisodium Oligosaccharides, Water
Questions or Comments?
Call toll free 1-855-548-4653
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
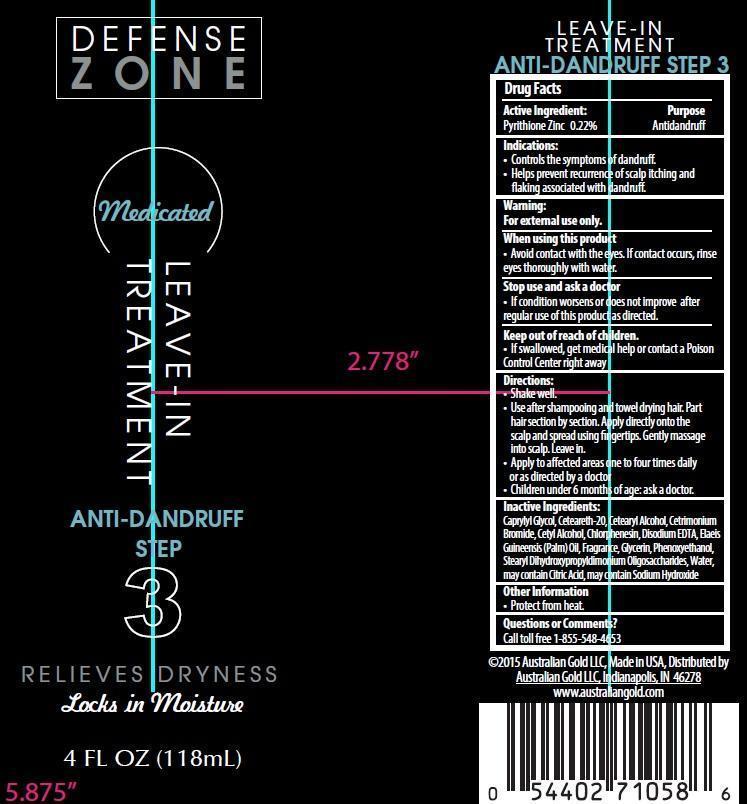
Defense
Zone
Medicated
LEAVE-IN
TREATMENT
Anti-Dandruff
Step
3
Relieves Dryness
Locks in Moisture
4 FL OZ (118mL)
Prime Enterprises, Inc.
