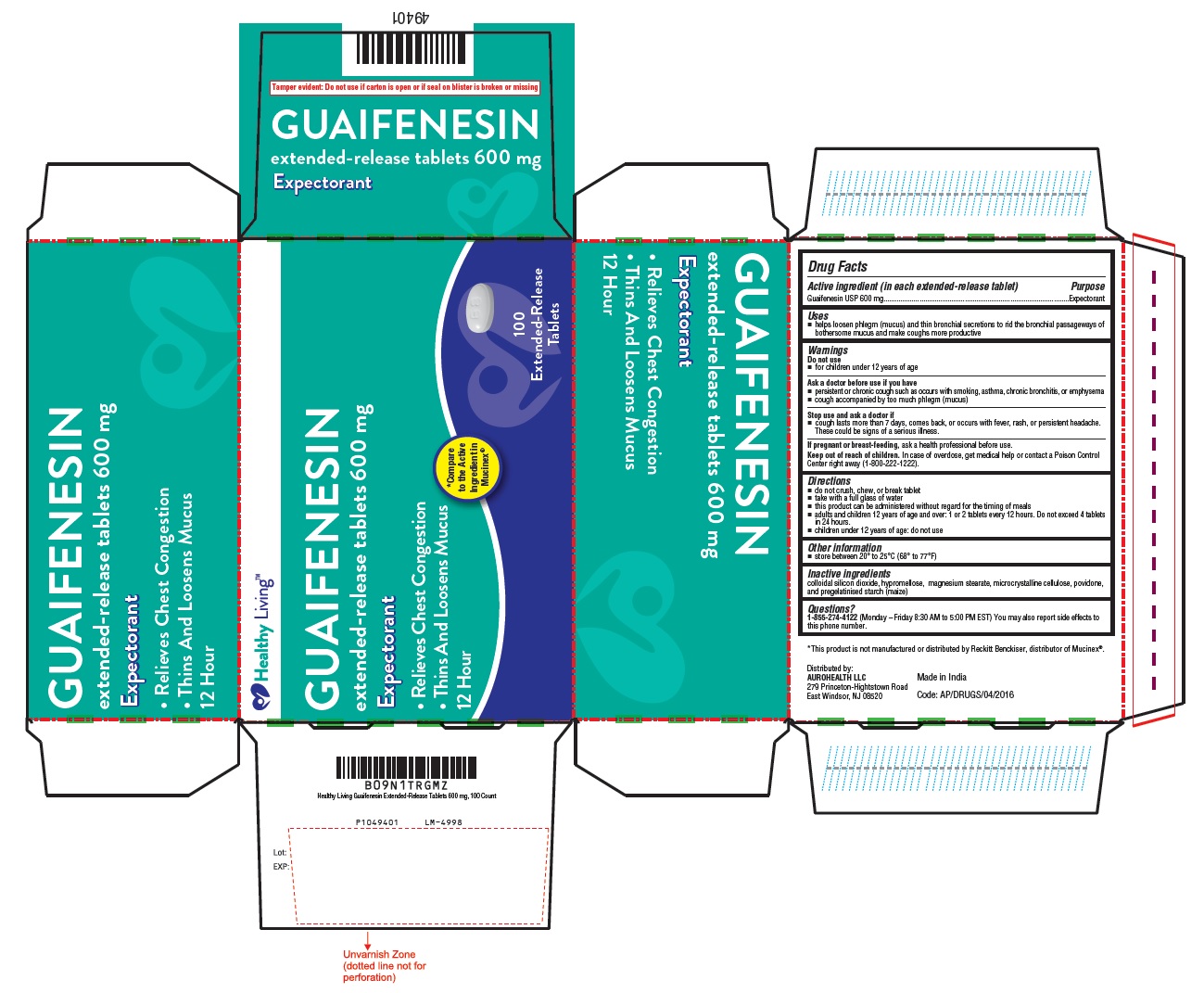
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours.
- children under 12 years of age: do not use
Inactive ingredients
colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, povidone and pregelatinised starch (maize)
Questions?
1-855-274-4122 (Monday – Friday 8:30 AM to 5:00 PM EST) You may also report side effects to this phone number.
Distributed by:
Aurohealth LLC
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Made in India
Code: AP/DRUGS/04/2016
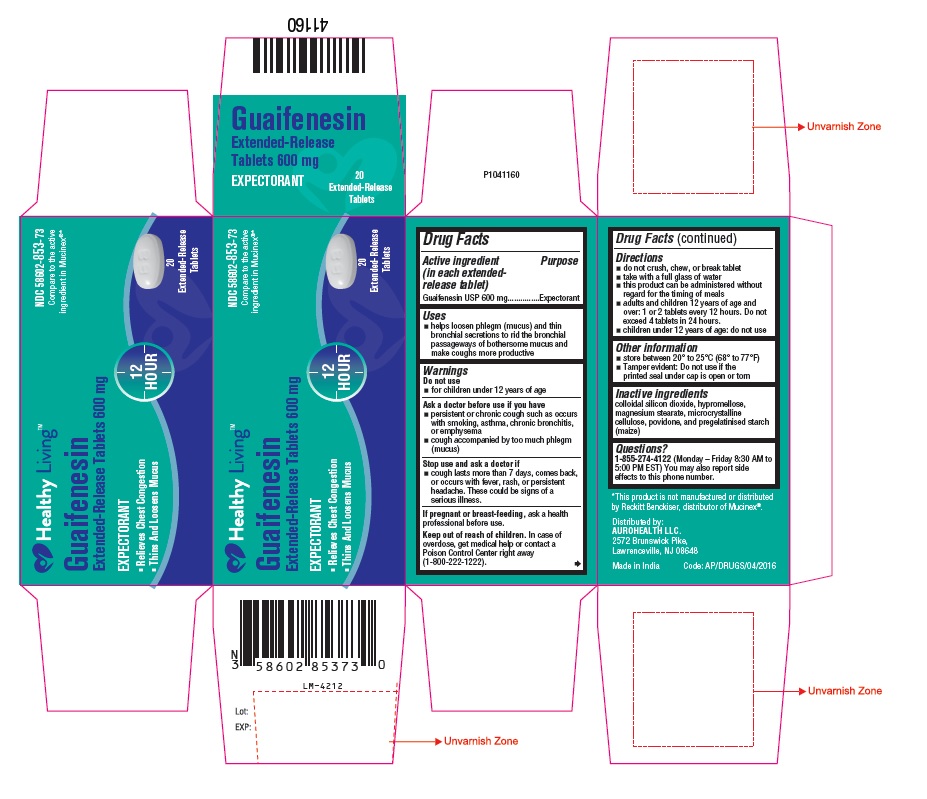
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 600 mg (20 Tablets Label)
Healthy Living™
NDC 58602-853-73
Guaifenesin
Extended-Release Tablets 600 mg
EXPECTORANT
12 HOUR
- Relieves Chest Congestion
- Thins And Loosens Mucus
20
Extended-Release
Tablets