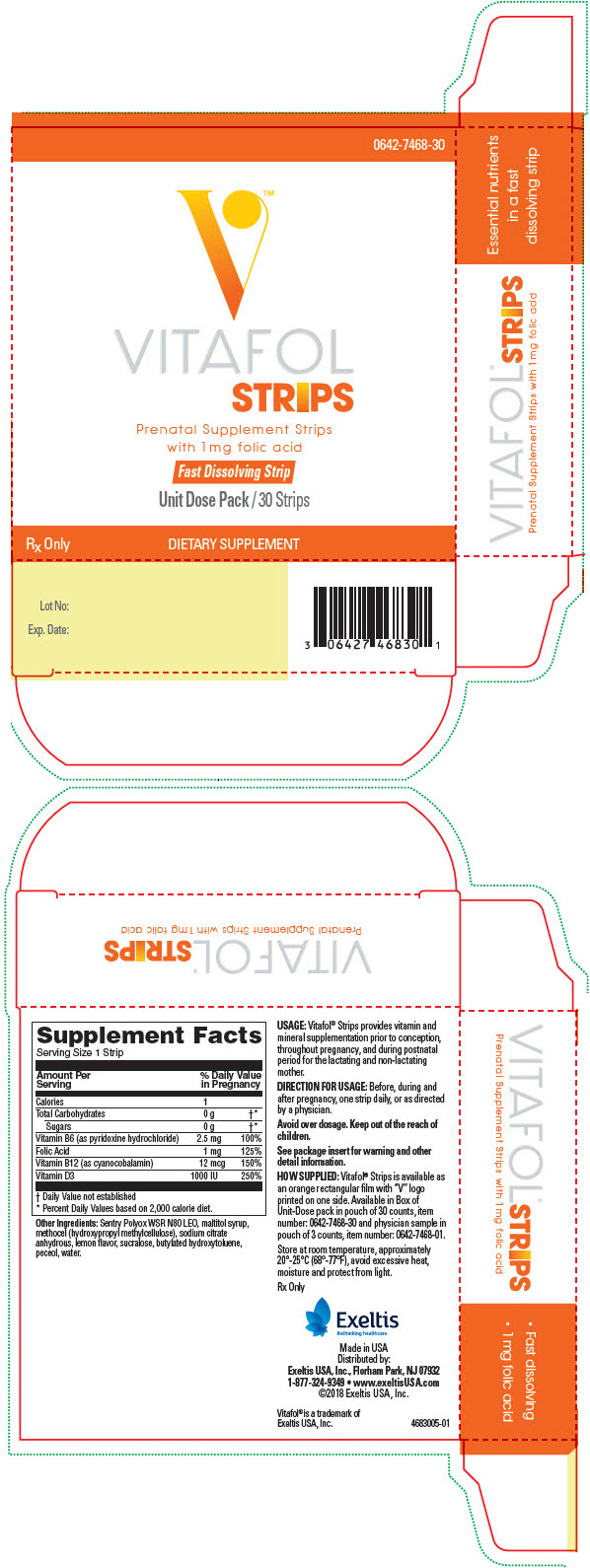
0642-7468-30
Rx Only
| These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. |
COMPOSITION
Amount per daily dose (1 strip)
| VITAMINS AND MINERALS: | |
|---|---|
| Calories | 1 |
| Total Carbohydrates | 0 g |
| Sugars | 0 g |
| Vitamin B6 (as pyridoxine hydrochloride) | 2.5 mg |
| Folic Acid | 1 mg |
| Vitamin B12 (as cyanocobalamin) | 12 mcg |
| Vitamin D (as cholecalciferol) | 1000 IU |
Other Ingredients:
Sentry Polyox WSR N80 LEO, maltitol syrup, methocel (hydroxypropyl methylcellulose), sodium citrate anhydrous, lemon flavor, sucralose, butylated hydroxytoluene, peceol, water.
USAGE
Vitafol® Strips provides vitamin and mineral supplementation prior to conception, throughout pregnancy, and postnatal period for the lactating and non-lactating women.
CONTRAINDICATIONS
Vitafol® Strips is contraindicated in patients with hypersensitivity to any of its components or color additives.
Folic acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (vitamin B12).
WARNINGS/PRECAUTIONS
This product is intended for use as directed by your healthcare provider. Please do not share with others.
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive.
The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Do not use if inner seal is broken or missing.
Do not exceed recommended dose.
Keep out of the reach of children.
DRUG INTERACTIONS
Medications for an overactive thyroid (anti-thyroid drugs) used in conjunction with iodine supplementation may lead to hypothyroidism.
Medications for hypertension used in conjunction with iodine supplementation may increase potassium.
High doses of folic acid may result in decreased serum levels of the anticonvulsant drugs; carbamazepine, fosphenytoin, phenytoin, phenobarbitol, valproic acid. Folic acid may decrease a patient's response to methotrexate.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Consult appropriate references for additional specific vitamin-drug interactions.
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals, but generally at doses substantially higher than those in Vitafol® Strips. However, allergic and idiosyncratic reactions are possible at any dose. Reported adverse events include skin ailments, gastrointestinal complaints, glucose abnormalities, and visual problems.
DIRECTIONS FOR USE
Before, during and after pregnancy, take one Vitafol® Strip daily, or as directed by a physician.
HOW SUPPLIED
Vitafol® Strips is available as an orange rectangular film with "V" logo printed on one side. Available in Box of Unit-Dose pack in pouch of 30 counts, Item No. 0642-7468-30 and as physician sample in pouch of 3 counts, Item No. 0642-7468-01.