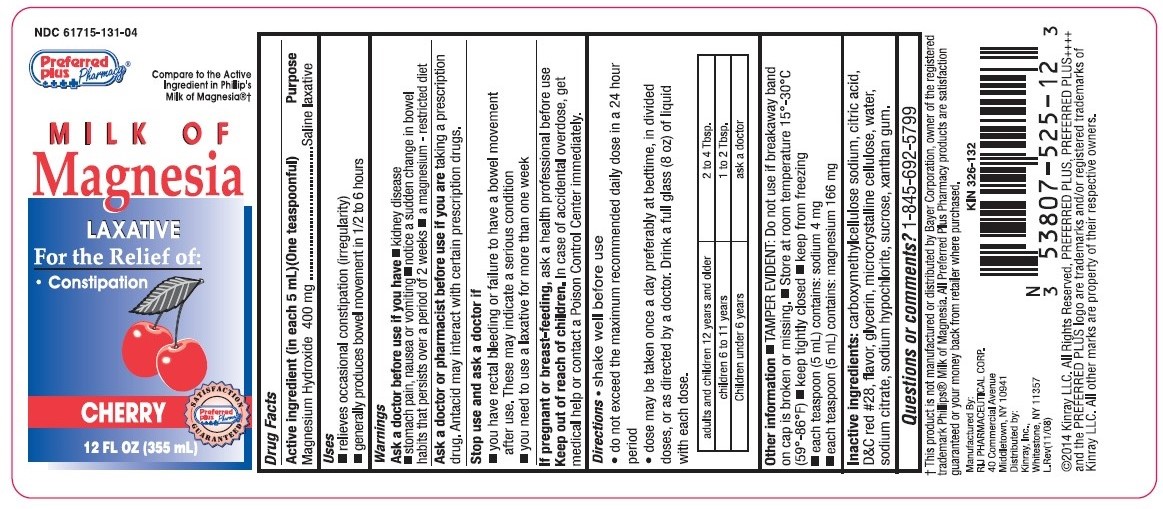
Use
- •
- relieves occasional constipation (irregularity).
- •
- This product generally produces bowel movement in ½ to 6 hours.
Warnings
Ask a doctor before use if you have
- •
- kidney disease
- •
- stomach pain, nausea, or vomiting
- •
- a sudden change in bowel habits that persist over a period of 2 weeks
- •
- a magnesium - restricted diet
Ask a doctor or pharmacist before use if you are
taking a prescription drug. This product may interact with certain prescription drugs.
Directions
- •
- shake well before use
- •
- do not exceed the maximum recommended daily dose in a 24 hour period
- •
- dose may be taken once a day preferably at bedtime, in divided doses, or as directed by a doctor. Drink a full glass (8 oz) of liquid with each dose
- •
- mL = milliliter, TBSP = Tablespoonful
|
adults and children 12 years and older |
2 to 4 Tbsp. |
|
children 6 to 11 years |
1 to 2 Tbsp. |
|
children under 6 years |
ask a doctor |
Other information
- •
- TAMPER EVIDENT: Do not use if breakaway band on cap is broken or missing.
- •
- Store at room temperature 15º - 30ºC (59º - 86ºF)
- •
- keep tightly closed
- •
- keep from freezing
- •
- each tablespoon (5 mL) contains: sodium 4 mg
- •
- each tablespoon (5 mL) contains: magnesium 166 mg