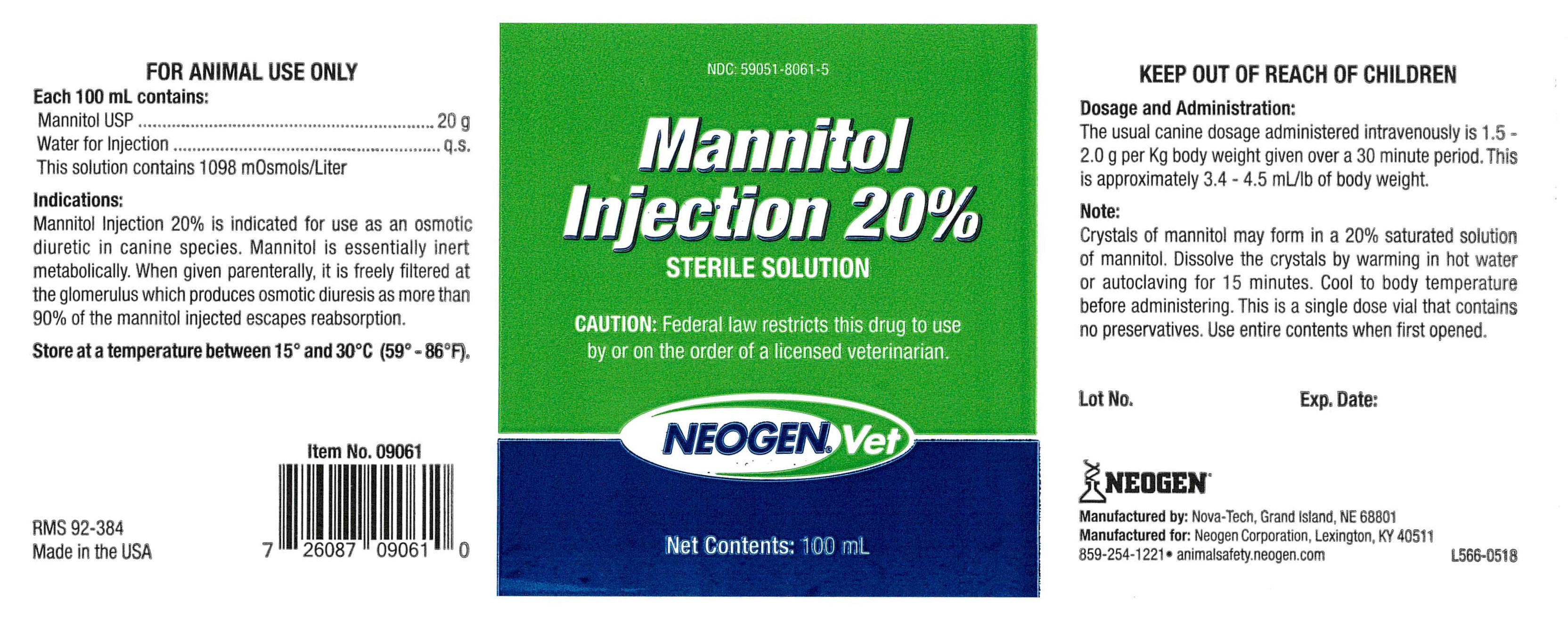
INDICATIONS:
Mannitol Injection 20% is indicated for use as an osmotic diuretic in canine species. Mannitol is essentially inert metabolically. When given parenterally, it is freely filtered at the glomerulus which produces osmotic diuresis as more than 90% of the mannitol injected escapes reabsorption.
Each 100 mL Contains:
Mannitol USP...................................20 g
Water for Injection...........................q.s.
This solution contains 1098 m0smols/Liter
Dosage and Administration:
The usual canine dosage administered intravenously is 1.5 - 2.0 g per Kg body weight given over a 30 minute period. This is approximately 3.4-4.5 mL/lb of body weight.
Note:
Crystals of mannitol may form in a 20% saturated solution of mannitol. Dissolve the crystals by warming in hot water or autoclaving for 15 minutes. Cool to body temperature before administering. This is a single dose vial that contains no preservatives. Use entire contents when first opened.
RMS 92-384
Made in the USA
Item No. 09061
NDC 59051-8061-5
Mannitol Injection 20%
Sterile Solution
NeogenVet
Net Contentes: 100 mL
Lot No.
Exp. Date:
Neogen
Manufactured by: Nova-Tech, Grand Island, NE 68801
Manufactured for: Neogen Corporation, Lexington, KY 40511
859-254-1221
animalsafety.neogen.com
L566-0518