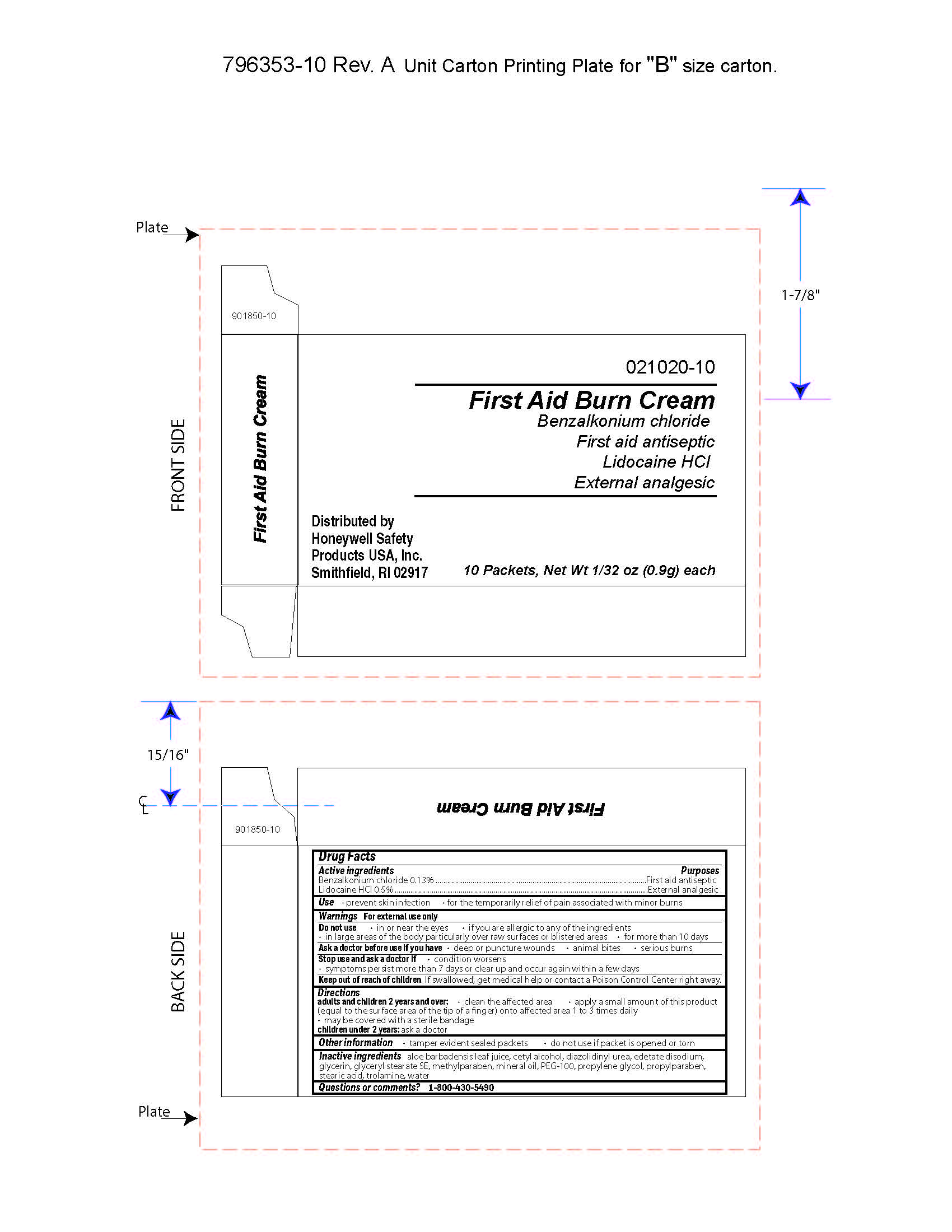
First Aid Burn Cream
Uses
- prevent skin infection
- for temporary relief of pain associated with minor burns
First Aid Burn Cream
Warnings
For external use only
First Aid Burn Cream
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
First Aid Burn Cream
Other information
- tamper evident sealed packets
- do not use if packet is opened or torn
First Aid Burn Cream
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
Povidone Iodine Swab
Active ingredient
Povidone-iodine solution USP, 10% (equivalent to 1% titratable iodine)
Povidone Iodine Swab
Uses
- first aid to help prevent the risk of infection in minor cuts, scrapes, and burns
Povidone Iodine Swab
Directions
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
Povidone Iodine Swab
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
Povidone Iodine Swab
Inactive ingredients
citric acid, disodium phosphate,nonoxynol-9, sodium hydroxide, water
4163
013055-4444 Kit Contents
1 FIRST AID BURN CREAM 6 PER
1 TRIANGULAR BDG, NON-STERILE
1 GAUZE PADS, 3" X 3", 4 PER
1 ADH TAPE, .5" X 2.5 YD, 2 PER
1 GAUZE COMP, 1 SQ YARD, 1 PER
1 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 PVP IODINE WIPES 10 PER
1 NITRILE GLOVES 2PR BBP
LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
1 LBL CONTS 6 3/4"X3 1/2" ID B
1 KIT STL 10 UN WHITE 01
4350
018502-4220 kit contents
1 TRIANGULAR BDG, NON-STERILE
1 GAUZE PADS, 3" X 3", 4 PER
1 GAUZE COMP, 18" X 36", 2 PER
1 TWEEZER PLASTICS 4"
4 O/H PAK,ADH BDG 2"X4", X-LG
1 FIRST AID GUIDE ASHI
10 PVP PREP PADS MEDIUM
1 SCISSOR BDGE 4" RED PLS HDL
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
1 1" X 3" PLASTIC BANDS 16/BAG
6 FIRST AID CREAM 1.0GR PKT EACH
2 TAPE ADHESIVE 1/2 X 2.5 125133
1 KIT BAG SOFT PACK SMALL
1 LBL CONTENTS ANSI Z308.1-2009 REV B
1 COLD PACK UNIT 4"X6" BULK
4 WOVEN FINGERTIP BANDAGE 2"
4 WOVEN KNUCKLE BANDAGE
4375
Z018502-4220
1 TRIANGULAR BDG, NON-STERILE
1 GAUZE PADS, 3" X 3", 4 PER
1 GAUZE COMP, 18" X 36", 2 PER
1 TWEEZER PLASTICS 4"
4 O/H PAK,ADH BDG 2"X4", X-LG
1 FIRST AID GUIDE ASHI
10 PVP PREP PADS MEDIUM
1 SCISSOR BDGE 4" RED PLS HDL
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
1 1" X 3" PLASTIC BANDS 16/BAG
6 FIRST AID CREAM 1.0GR PKT EACH
2 TAPE ADHESIVE 1/2 X 2.5 125133
1 KIT BAG SOFT PACK SMALL
1 LBL CONTENTS ANSI Z308.1-2009 REV B
1 COLD PACK UNIT 4"X6" BULK
4 WOVEN FINGERTIP BANDAGE 2"
4 WOVEN KNUCKLE BANDAGE