Warnings
For external use only- Obtain immediate medical treatment for all open wounds in or near the eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
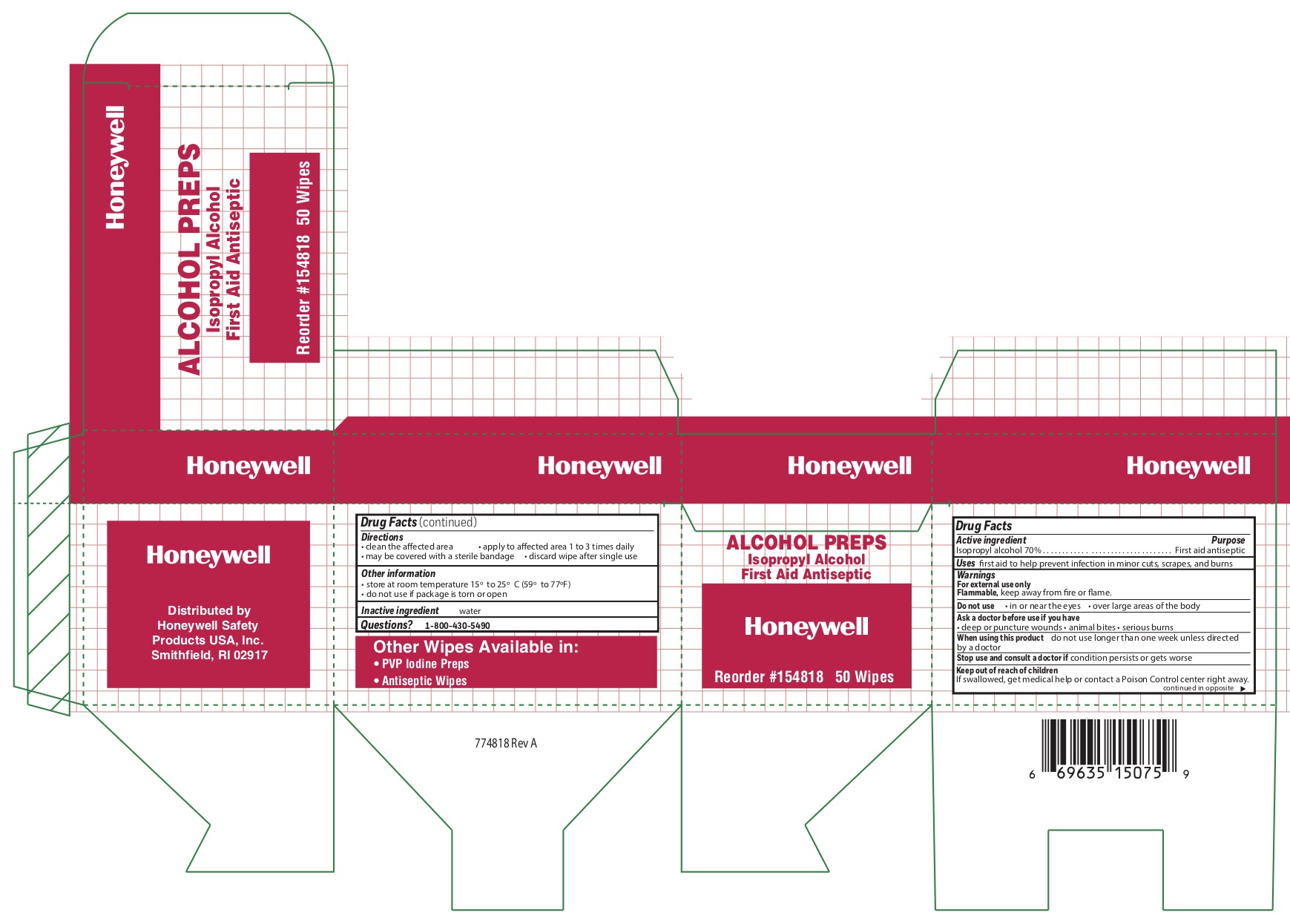
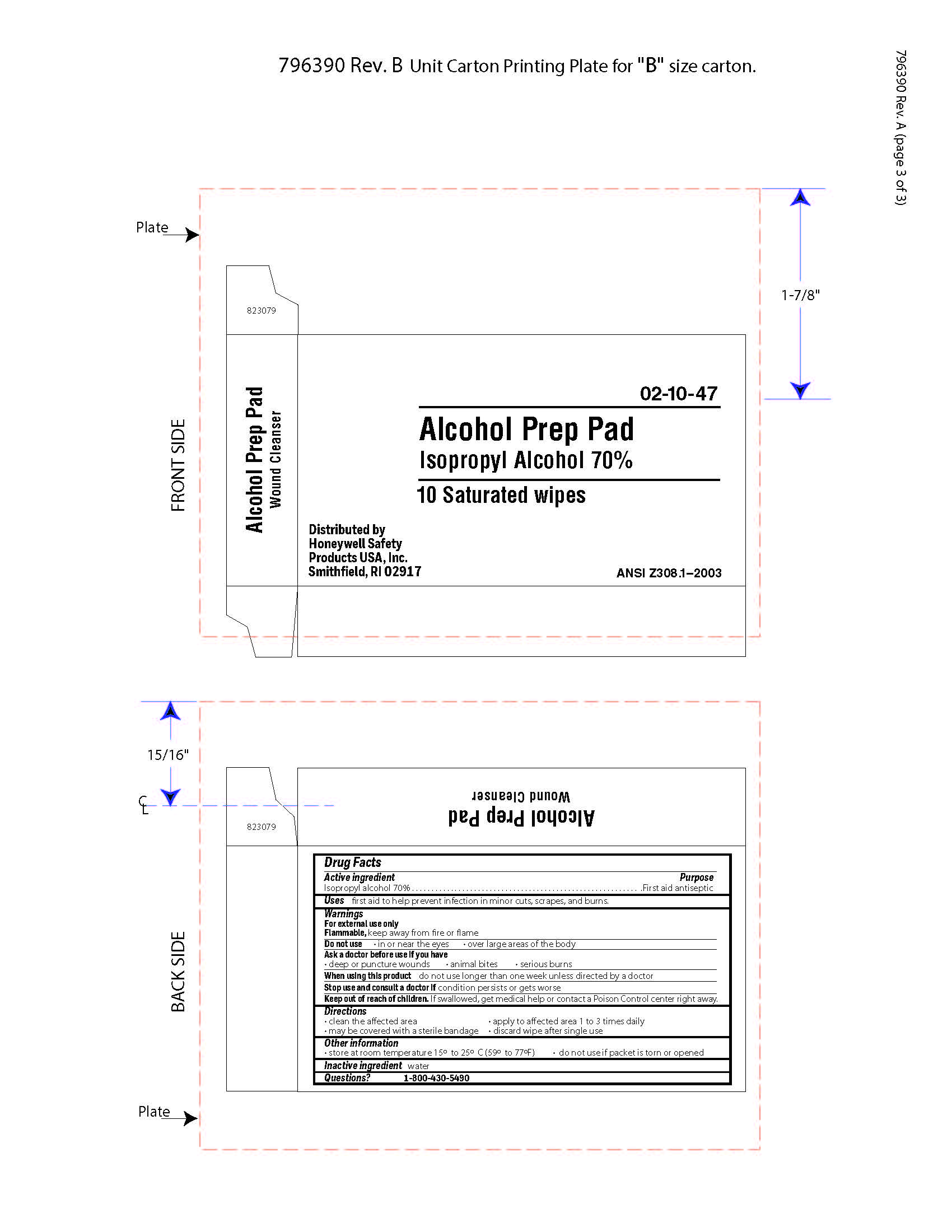
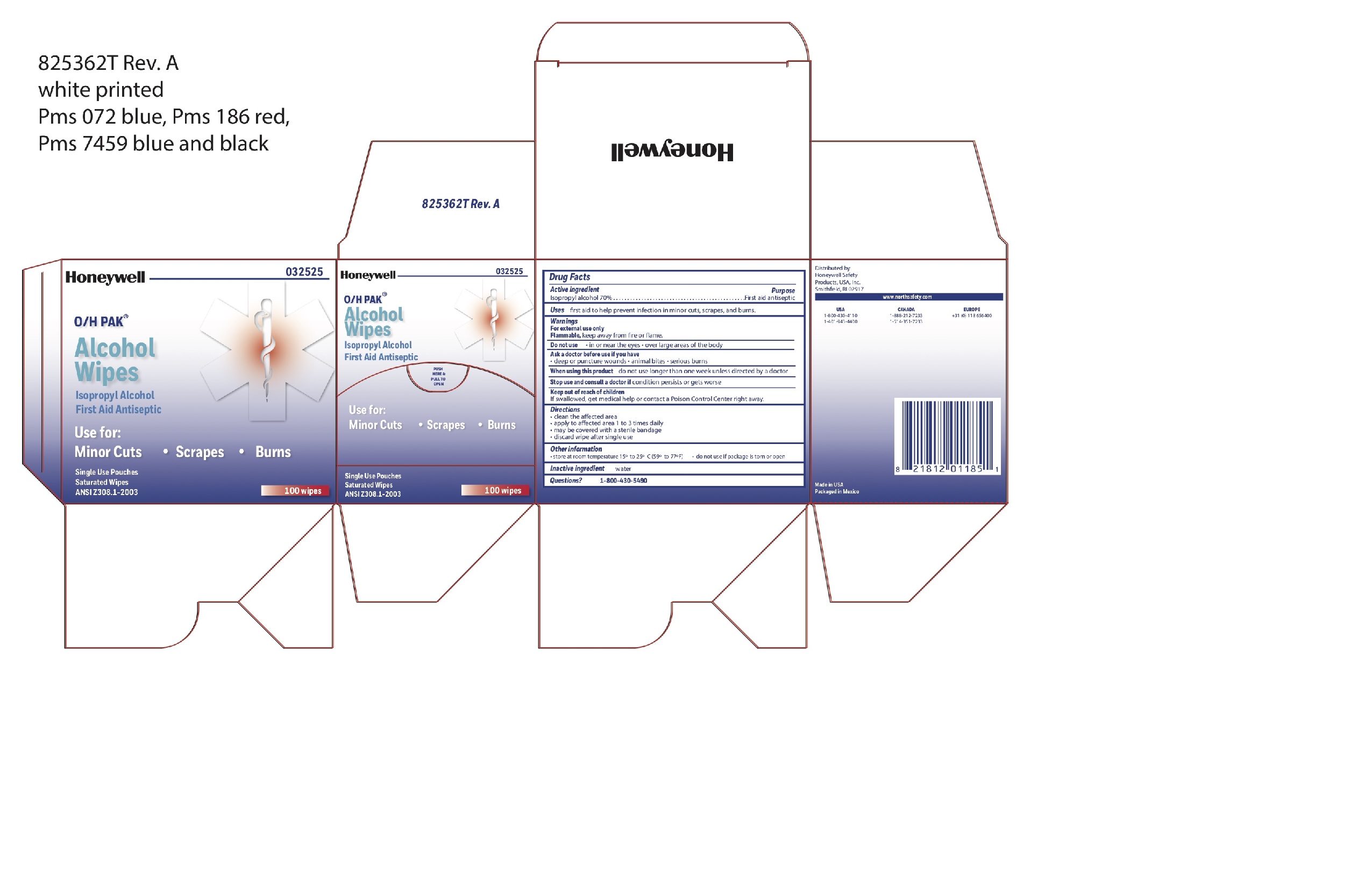
Alcohol Prep
Directions
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily -
- discard wipe after single use
Alcohol Prep
Other information
- store at room temperature 15 0 to 25 0 C (59 0 to 77 0 F)
- do not use if packet is torn or opened
4169
010068-4509 Kit Contents
1 KNUCKLE BAND 8 PER
1 GAUZE BANDAGE, 4" X 6 YD
1 TRIANGULAR BDG, NON-STERILE
1 GAUZE PADS, 3" X 3", 4 PER
1 ADH TAPE, .5" X 2.5 YD, 2 PER
1 FORCEPS & SCISSORS, 1 EA
1 INSTANT COLD PACK 4" X 6"
1 BANDAGE COMP, 3" OFFSET, 2 PER
1 1 OZ EYE WASH W/PADS & STRIPS
1 ALCOHOL PREP PADS 10P
2 ADH BDG, CLOTH, 1"X3", 16 PER
1 MICROSHIELD W/VNL GLV/ALCL
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 16 UN (HORIZONTAL)
1 LABL INSTR FA REV A
4366
SF00004168 kit contents
1 RESCUE BLANKET 1 PER
1 FINGERTIP BANDAGE, 10 PER
1 ALCOHOL PREP PADS 10P
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 ADH BDG, CLOTH, 1"X3", 16 PER
1 ELASTIC ROLLED GZ 3" ST
1 ELASTIC ROLLED GZ 4" ST
1 BLOODSTOPPER
2 ABD COMBINE PAD 5" X 9"
1 GZE PADS STERILE 3"X 3" 10'S
2 ABD PADS 8"X10" STERILE
1 ELASTIC BANDAGE 3" X 4.5YD
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR LISTER BDG S/S 5 1/2"
1 ISO-SHIELD CPR ADULT/CHLD 1BG
LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
1 EZ PROTECTION KIT
1 MOIST TOWELETTES 10 IN ZIP BAG
1 EMERGENCY MED FANNY PACK EMPTY
1 SOLIDIFIER BBP POUCH 2 OZ ID E
1 CORRUGATED WIPES RSC
2 COLD PACK 5"X 9" BULK
1 TRI BNDG NON WOVEN 40"X40"X56"
1 KNUCKLE BANDS 16'S
4368
SF00004568 kit contents
1 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 ALCOHOL PREP PADS 10P
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 FIRST AID GUIDE ASHI
1 EMERGENCY SURVIVAL BLANKET
1 TAPE ADHESIVE 1"X 5 YD PLSTC
2 GAUZE CLEAN-WRAP BDGE N/S 3"
1 GAUZE CLEAN-WRAP BDGE N/S 4"
2 ABD COMBINE PAD 5" X 9"
1 GZE PADS STERILE 4"X 4" 10'S
1 GZE PADS STERILE 3"X 3" 25'S
1 MULTI-TRAUMA DRESSING 12"X30"
1 ELASTIC BANDAGE 3" X 4.5YD
1 ELASTIC BANDAGE 4" X 4.5YD
1 MICROSHIELD BAGGED 72-151
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 EMPTY BAG RED 12X12X10
1 SAM SPLINT 4.5"X36" ORNGE/BL
LBL STOCK 6-3/8"X4"
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
10 PR LRG NITRILE GLVES
1 WATER-JEL BURN DRESSING 4 X 4
8 CORNER STYROFOAM 3X3X3
2 TRI BNDG NON WOVEN 40"X40"X56"
2 COLD PACK UNIT 4"X6" BULK
4 EYE PADS STD OVAL STERILE