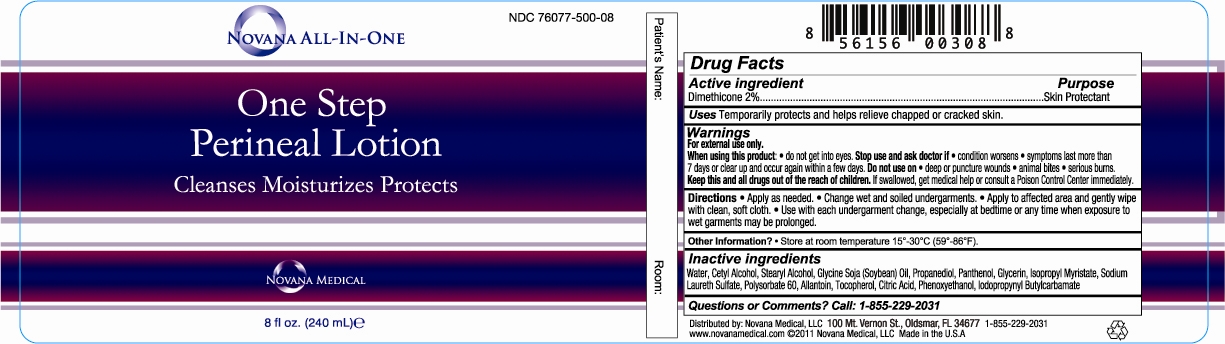
NOVANA ALL-IN-ONE ONE STEP PERINEAL - dimethicone lotion
NOVANA MEDICAL LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient Purpose
Dimethicone 2%.......................................................................................................Skin Protectant
Uses Temporarily protects and helps relieve chapped or cracked skin.
Warnings
For external use only.
When using this product • Avoid contact with eyes • Stop use and ask doctor if • If condition worsens •symptoms last more than 7 days or clear up and occur again within a few days. Do not use on •deep or puncture wounds •animal bites •serious burns.
Keep this and all drugs out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions •Apply as needed. • Change wet and soiled undergarments. • Apply to affected area and gently wipe with clean, soft cloth. • Use with each undergarment change, especially at bedtime or any time when exposure to wet garments may be prolonged.
Inactive ingredients
Water, Cetyl Alcohol, Stearyl Alcohol, Glycine Soja (Soybean) Oil, Propanediol, Polysorbate 60,
Panthenol, Glycerin, Isopropyl Myristate, Allantoin, Tocopherol, Sodium Laureth Sulfate, Citric Acid,
Phenoxyethanol, Iodopropynyl Butylcarbamate