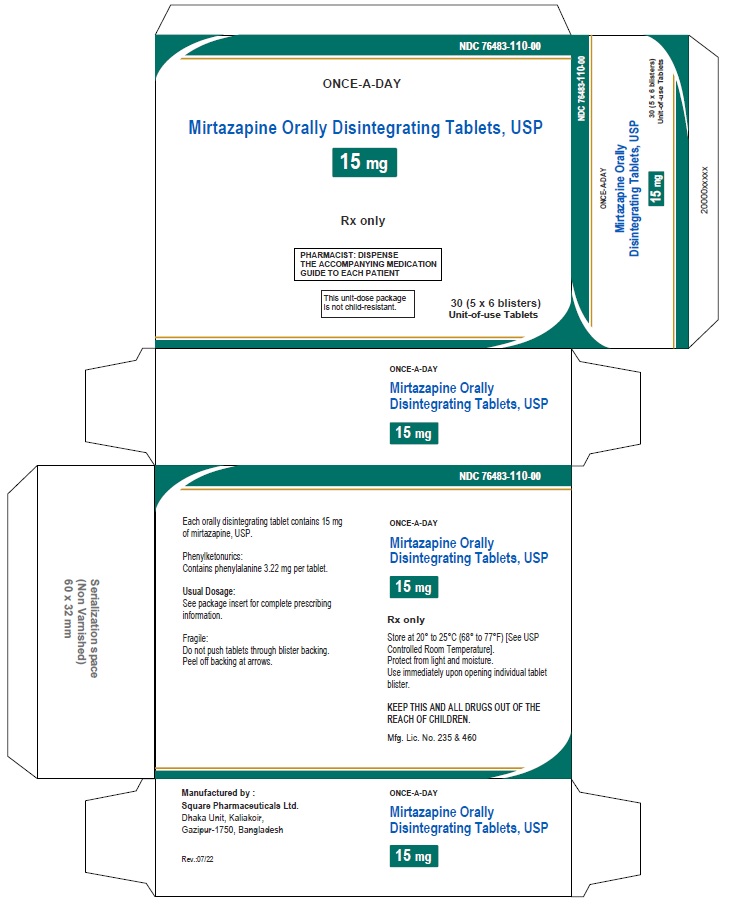
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 76483-110-00 in unit-dose blister cartons of 30 (5 x 6 blisters) Unit-of-use Tablets
Mirtazapine Orally Disintegrating Tablets USP, 15 mg
Rx only
30 Tablets
NDC 76483-111-00 in unit-dose blister cartons of 30 (5 x 6 blisters) Unit-of-use Tablets
Mirtazapine Orally Disintegrating Tablets USP, 30 mg
Rx only
30 Tablets

NDC 76483-112-00 in unit-dose blister cartons of 30 (5 x 6 blisters) Unit-of-use Tablets
Mirtazapine Orally Disintegrating Tablets USP, 45 mg
Rx only
30 Tablets
