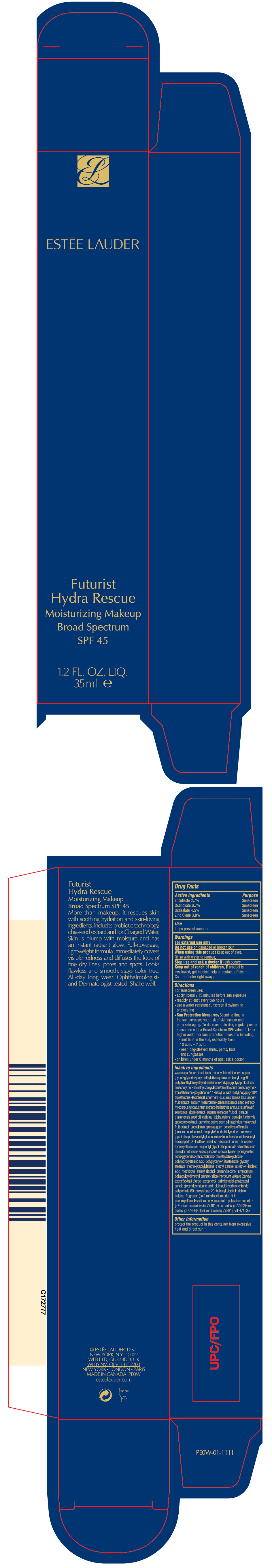
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
Inactive ingredients
water\aqua\eau • dimethicone • phenyl trimethicone • butylene glycol • glycerin • polymethylsilsesquioxane • lauryl peg-9 polydimethylsiloxyethyl dimethicone • hdi/ppg/polycaprolactone crosspolymer • trimethylsiloxysilicate/dimethiconol crosspolymer • tromethamine • polysilicone-11 • hexyl laurate • cetyl peg/ppg-10/1 dimethicone • lactobacillus ferment • cucumis sativus (cucumber) fruit extract • sodium hyaluronate • salvia hispanica seed extract • hylocereus undatus fruit extract • helianthus annuus (sunflower) seedcake • algae extract • euterpe oleracea fruit oil • carapa guaianensis seed oil • caffeine • jojoba esters • tremella fuciformis sporocarp extract • camelina sativa seed oil • sapindus mukorossi fruit extract • caesalpinia spinosa gum • copaifera officinalis (balsam copaiba) resin • caprylic/capric triglyceride • propylene glycol dicaprate • acetyl glucosamine • tocopheryl acetate • acetyl hexapeptide-8 • lecithin • trehalose • disteardimonium hectorite • hydroxyethyl urea • neopentyl glycol diheptanoate • dimethicone/divinyldimethicone silsesquioxane crosspolymer • hydrogenated coco-glycerides • phospholipids • trimethylsiloxysilicate • polyhydroxystearic acid • polyglyceryl-4 isostearate • glyceryl stearate • triethoxycaprylylsilane • triethyl citrate • laureth-7 • linoleic acid • methicone • stearyl alcohol • cetearyl alcohol • ammonium polyacryloyldimethyl taurate • silica • hordeum vulgare (barley) extract\extrait d'orge • tocopherol • palmitic acid • phytosteryl canola glycerides • stearic acid • oleic acid • sodium chloride • polysorbate 80 • polysorbate 20 • behenyl alcohol • triolein • betaine • fragrance (parfum) • disodium edta • bht • phenoxyethanol • sodium dehydroacetate • potassium sorbate • [+/- mica • iron oxides (ci 77491) • iron oxides (ci 77492) • iron oxides (ci 77499) • titanium dioxide (ci 77891)] <iln47153>