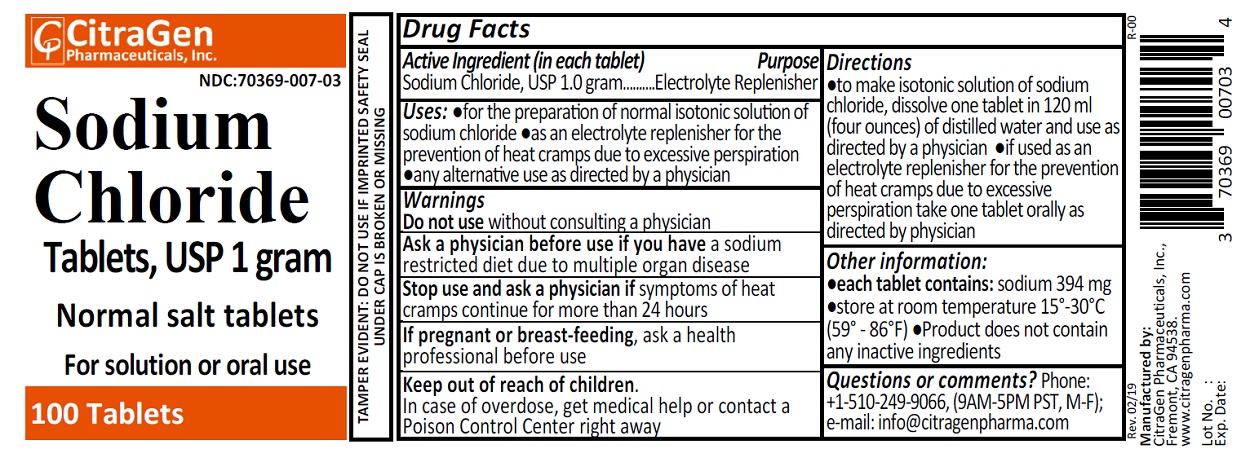
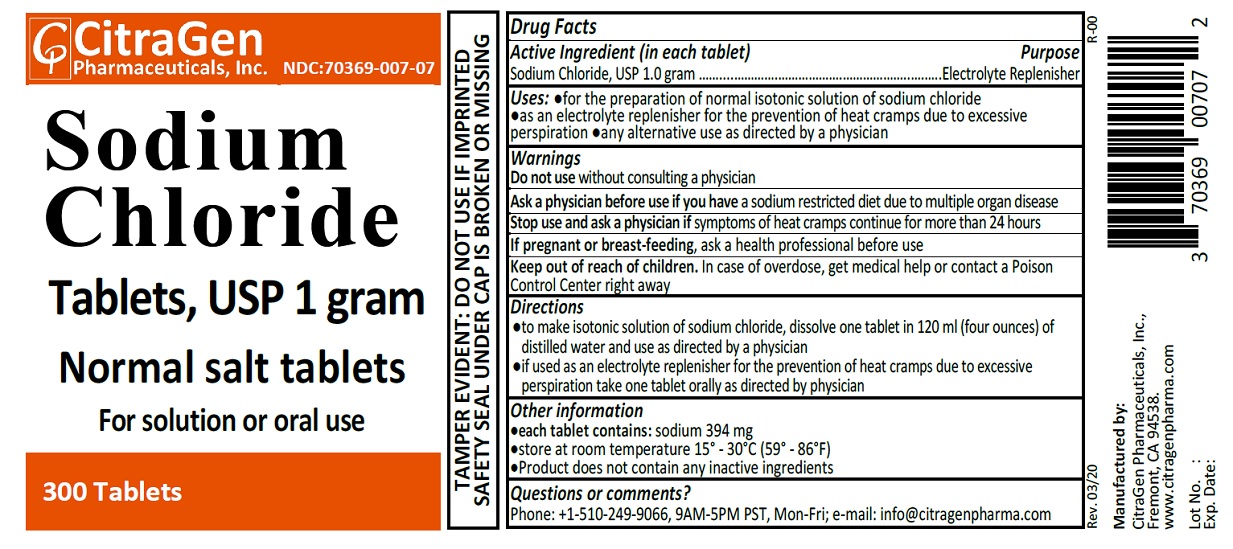
Uses
- for the preparation of normal isotonic solution of sodium chloride
- as an electrolyte replenisher for the prevention of heat cramps due to excessive perspiration
- any alternative use as directed by a physician
Directions
- to make isontonic solution of sodium chloride, dissolve one tablet in 120 ml (four ounces) of distilled water and use as directed by a physician.
- if used as an electrolyte replenisher for the prevention of heat cramps due to excessive perspiration take one tablet orally as directed by your physician.
Other information:
- each tablet contains: sodium 394 mg
- store at room temperature 15°-30°C (59°-86°F)
- product does not contain any inactive ingredients
Questions or comments?
Phone: +1-510-249-9066 (9AM-5PM PST, Mon-Fri); e-mail: info@citragenpharma.com
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Manufactured by:
CitraGen Pharmaceuticals, Inc.,
Fremont, CA 94538.
www.citragenpharma.com
Rev. 02/19 R-00
CitraGen Pharmaceuticals, Inc.
NDC: 70369‐007‐02
Sodium Chloride Tablets, USP 1 gram
Normal salt tablets
For solution or oral use
500 Tablets
THIS PACKAGE IS FOR HOUSEHOLDS WITHOUT YOUNG CHILDREN