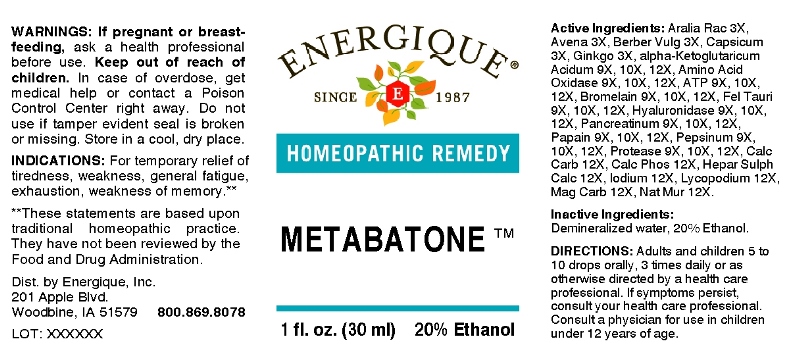
METABATONE- aralia racemosa, avena sativa, berberis vulgaris, capsicum annuum, ginkgo biloba, adenosinum triphosphoricum dinatrum, alpha-ketoglutaricum acidum, amino acids, bromelain, fel tauri, hyaluronidase, pancreatinum, papain, pepsinum, protease, calcarea carbonica, calcarea phosphorica, hepar sulphuris calcareum, iodium, lycopodium clavatum, magnesia carbonica, natrum muriaticum liquid
Energique, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Aralia Racemosa 3X, Avena Sativa 3X, Berberis Vulgaris 3X, Capsicum Annuum 3X, Ginkgo Biloba 3X, Adenosinum Triphosphoricum Dinatrum 9X, 10X, 12X, Alpha-Ketoglutaricum Acidum 9X, 10X, 12X, Amino Acids 9X, 10X, 12X, Bromelain 9X, 10X, 12X, Fel Tauri 9X, 10X, 12X, Hyaluronidase 9X, 10X, 12X, Pancreatinum 9X, 10X, 12X, Papain 9X, 10X, 12X, Pepsinum 9X, 10X, 12X, Protease 9X, 10X, 12X, Calcarea Carbonica 12X, Calcarea Phosphorica 12X, Hepar Sulphuris Calcareum 12X, Iodium 12X, Lycopodium Clavatum 12X, Magnesia Carbonica 12X, Natrum Muriaticum 12X.
INDICATIONS:
For temporary relief of tiredness, weakness, general fatigue, exhaustion, weakness of memory.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist, consult your health care professional. Consult a physician for use in children under 12 years of age.
| METABATONE
aralia racemosa, avena sativa, berberis vulgaris, capsicum annuum, ginkgo biloba, adenosinum triphosphoricum dinatrum, alpha-ketoglutaricum acidum, amino acids, bromelain, fel tauri, hyaluronidase, pancreatinum, papain, pepsinum, protease, calcarea carbonica, calcarea phosphorica, hepar sulphuris calcareum, iodium, lycopodium clavatum, magnesia carbonica, natrum muriaticum liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Energique, Inc. (789886132) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(44911-0296) , api manufacture(44911-0296) , label(44911-0296) , pack(44911-0296) | |