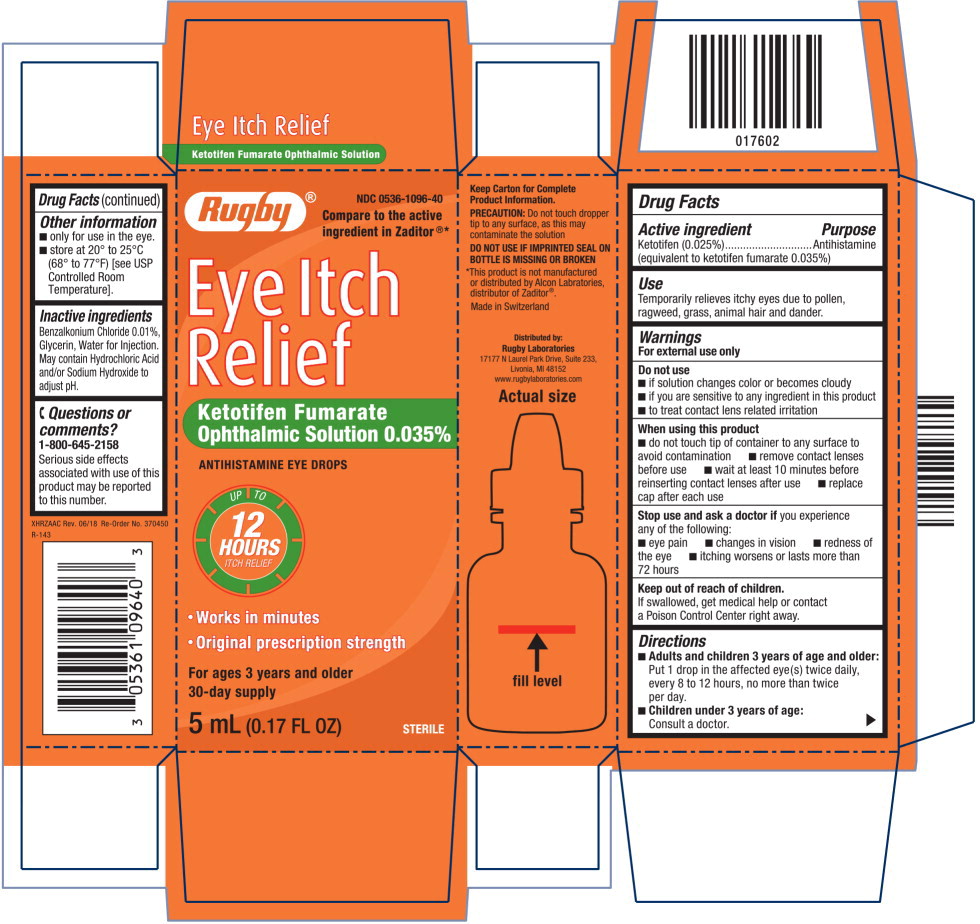
Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
Directions
-
Adults and children 3 years of age and older:
Put 1 drop in the affected eye(s) twice daily, every 8 to 12 hours, no more than twice per day. -
Children under 3 years of age:
Consult a doctor.
Other information
- only for use in the eye.
- store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Inactive ingredients
Benzalkonium Chloride 0.01%, Glycerin, Water for Injection. May contain Hydrochloric Acid and/or Sodium Hydroxide to adjust pH.
Questions or comments?
1-800-645-2158
Serious side effects associated with use of this product may be reported to this number
Principal Display Panel Text for Container Label:
Rugby® Logo
NDC 0536-1096-40
Eye Itch Relief
Ketotifen Fumarate
Ophthalmic Solution 0.035%
ANTIHISTAMINE EYE DROPS
5 mL (0.17 FL OZ) STERILE
Principal Display Panel Text for Carton Label:
Rugby® Logo NDC 0536-1096-40
Compare to the active
ingredient in Zaditor®*
Eye Itch
Relief
Ketotifen Fumarate
Ophthalmic Solution 0.035%
ANTIHISTAMINE EYE DROPS
UP TO
12
HOURS
ITCH RELIEF
- Works in minutes
- Original prescription strength
For ages 3 years and older
30-day supply
5 mL (0.17 FL OZ) STERILE