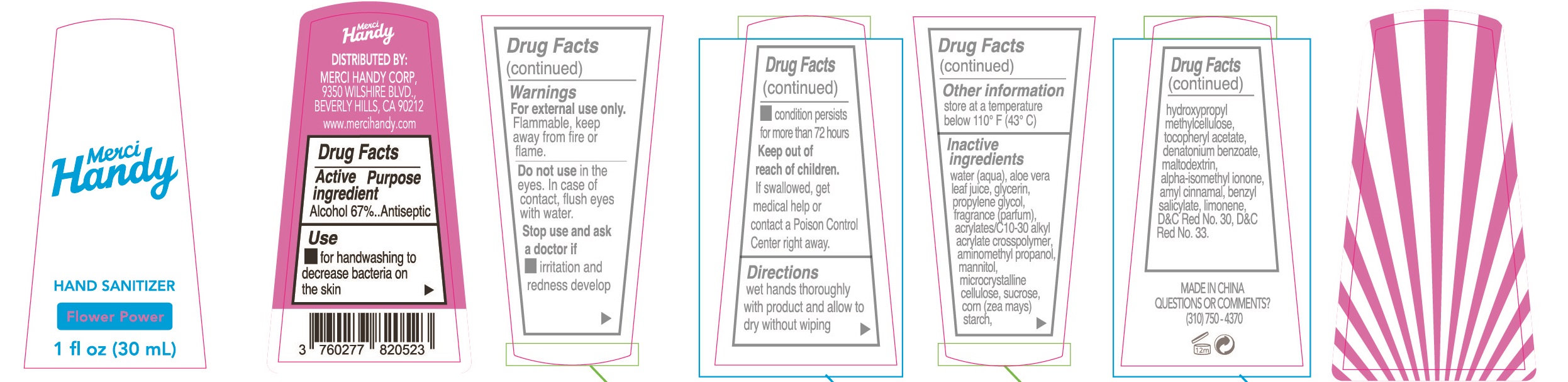
Warnings
For external use only. Flammable, keep away from fire or flame.
Do not use in the eyes.In case of contact, flush eyes with water.
Stop use and ask a doctor if
- irritation and redness develop.
- condition persists for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
water(aqua), aloe vera leaf juice, glycerin, propylene glycol, fragrance(parfum), acrylates/C30-10 alkyl acrylate crosspolymer, aminomethyl propanol, mannitol, microcrystalline cellulose, sucrose, corn(zea mays) starch, hydroxpropyl methyl cellulose, tocopheryl acetate, denatonium benzonate, maltodextrin, alpha-isomethyl ionone, amyl cinnamal, benzyl salicylate, limonene, D&C Red No.30, D&C Red No.33.