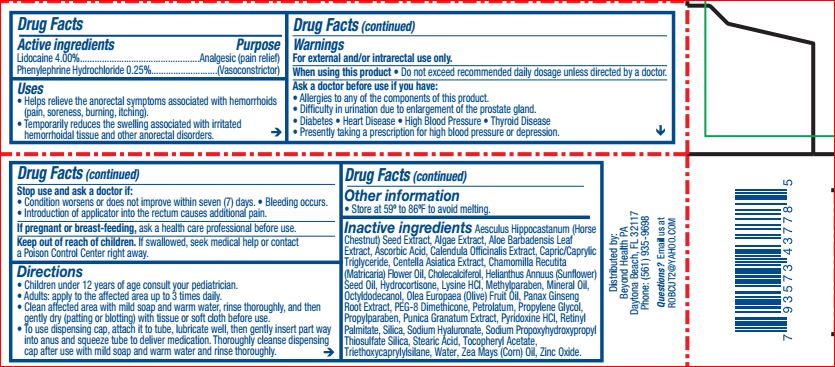
Helps relieve the anorectal symptoms associated with hemorrhoids (pain, soreness, burning, itching). Temporarily reduces the swelling associated with irritated hemorrhoidal tissue and other anorectal disorders.
For external and/or intrarectal use only.
When using this product do not exceed recommended daily dosage unless directed by a doctor.
Ask a doctor before use if you have allergies to any of the components of this product. Difficulty in urination due to enlargement of the prostate gland. Diabetes, heart disease, high blood pressure, thyroid disease. Presently taking a prescription for high blood pressure or depression.
Stop use and ask a doctor if condition worsens or does not improve within seven days, bleeding occurs. Introduction of applicator into the rectum causes additional pain.
If pregnant or breastfeeding, ask a health professional before use.
Children under 12 years of age consult your pediatrician.
Adults: apply to affected area up to 3 times daily.
Clean affected area with mild soap and warm water, rinse thoroughly, and then gently dry (patting or blotting) with tissue or soft cloth before use.
To use dispensing cap, attach it to tube, lubricate well then gently insert part way into anus and squeeze tube to deliver medication. Thoroughly cleanse dispensing cap after use with mild soap and warm water and rinse thoroughly.