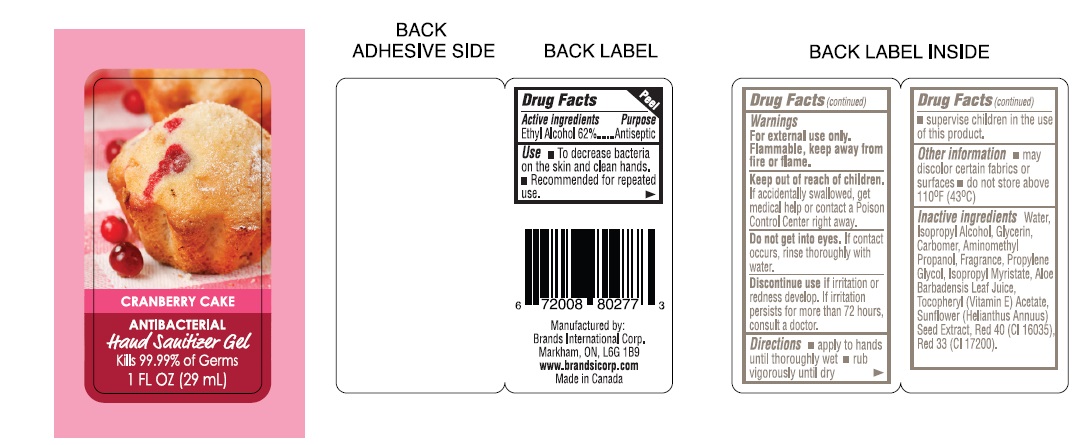
CRANBERRY CAKE- antibacterial hand sanitizer gel
Brands International
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CRANBERRY CAKE ANTIBACTERIAL HAND SANITIZER
ACTIVE INGREDIENT: ETHYL ALCOHOL 62%
PURPOSE ANTISEPTIC
USES: TO DECREASE BACTERIA ON THE SKIN AND CLEAN HANDS.
RECOMMENDED FOR REPEATED USE
WARNING: FOR EXTERNAL USE ONLY. FLAMMABLE,
KEEP AWAY FROM FIRE OR FLAME.
DO NOT GET INTO EYES. IF CONTACT OCCURS,RINSE THROUGHLY WITH WATER.
DISCONTINUE USE IF IRRITATION OR REDNESS DEVELOP. IF IRRITATION PERSISTS FOR MORE THEN 72 HOURS, CONSULT A DOCTOR.
SUPERVISE CHILDREN IN THE USE OF THIS PRODUCT.
OTHER INFORMATIONMAY DISCOLOR CERTAIN FEBRICS OR SURFACES.
INACTIVE INGREDIENTS: WATER, PISOPROPYL ALCOHOL, GLYCERIN, CARBOMER, AMINOMETHYL PROPANOL, PROPYLENE GLYCOL, ISOPROPYL MYRISTATE, ALOE BARBADENSIS LEAF JUICE, TOCOPHEROL ACETATE, SUNFLOWER (HELIANTHUS ANNUUS) SEED EXTRACT, MAY CONTAIN: FD&C BLUE NO. 1(CI 42090), FD&C RED NO. 40(CI 16035), FD&C YELLOW NO. 5 (CI 19140)
CRANBERRY CAKE ANTIBACTERIAL HAND SANITIZER