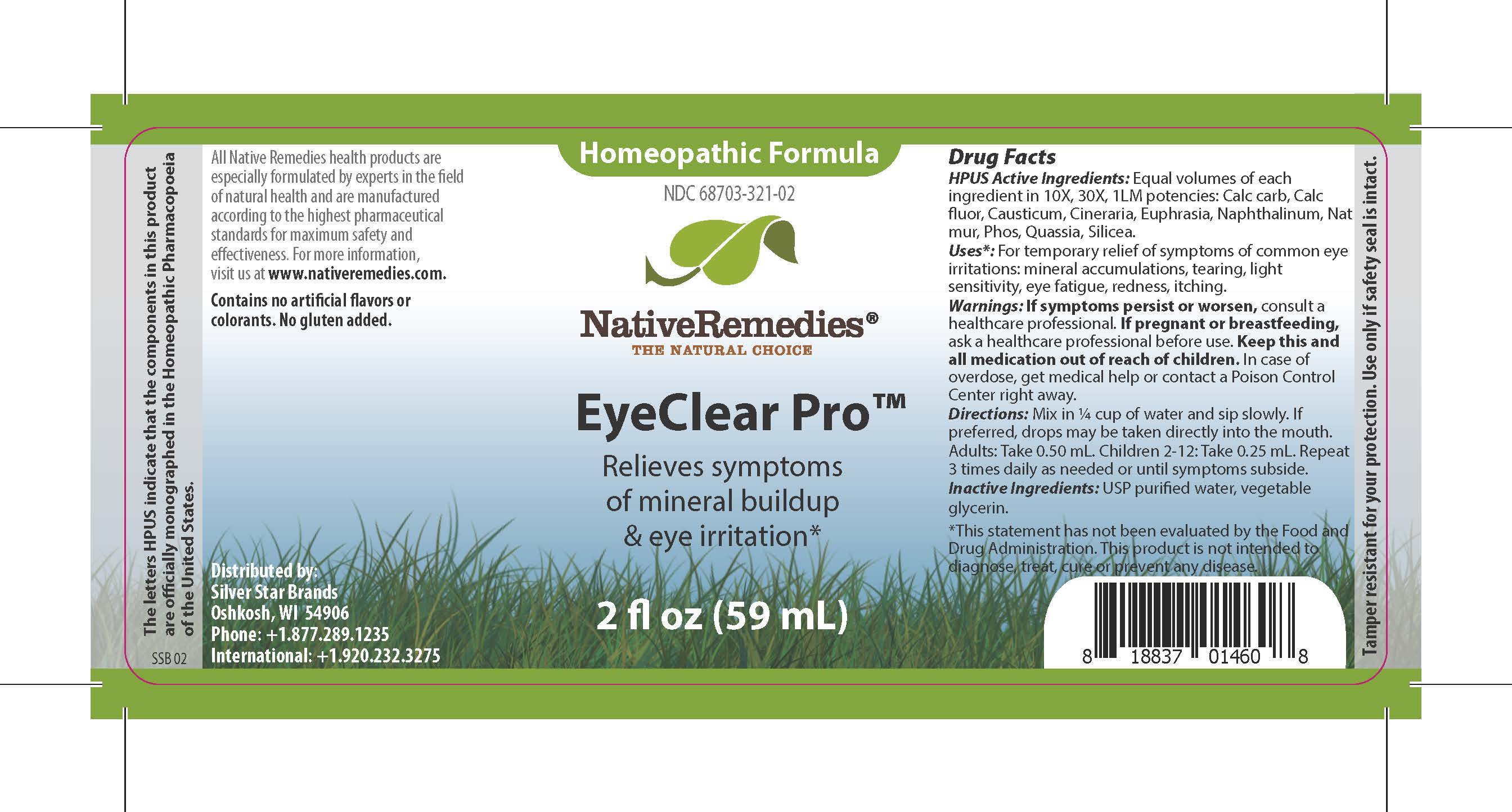
HPUS Active Ingredients
HPUS Active Ingredients: Equal volumes of each ingredient in 10X, 30X, 1LM potencies: Calc carb, Calc fluor, Causticum, Cineraria, Euphrasia, Naphthalinum, Nat mur, Phos, Quassia, Silicea.
The letters HPUS indicate that the components in this product are offically monographed in the Homeopathic Pharmacopoeia of the United States.
Uses*
Uses*: For temporary relief of symptoms of common eye irritations: mineral accumulations, tearing, light sensitivity, eye fatigue, redness, itching.
*This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Warnings
Warnings: If symptoms persist or worsen, consult a healthcare professional. If pregnant or breastfeeding, ask a healthcare professional before use. Keep this and all medication out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Directions: Mix in 1/4 cup of water and sip slowly. If preferred, drops may be taken directly into the mouth. Adults: Take 0.50 mL. Children 2-12: Take 0.25 mL. Repeat 3 times daily as needed or until symptoms subside.