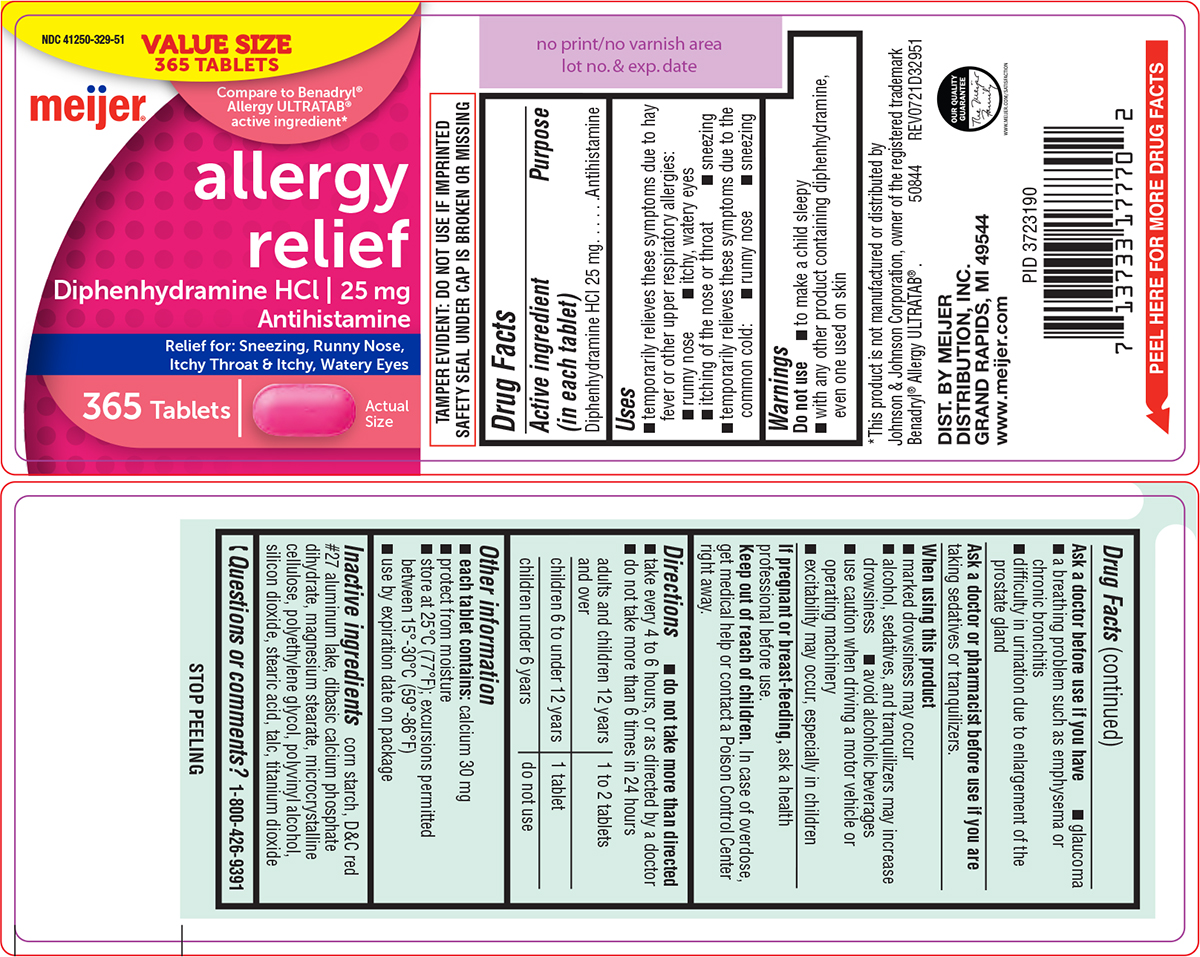
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- itching of the nose or throat
- sneezing
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
Directions
- do not take more than directed
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
| adults and children 12 years and over | 1 to 2 tablets |
| children 6 to under 12 years | 1 tablet |
| children under 6 years | do not use |
Other information
- each tablet contains: calcium 30 mg
- protect from moisture
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- use by expiration date on package
Inactive ingredients
corn starch, D&C red #27 aluminum lake, dibasic calcium phosphate dihydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, stearic acid, talc, titanium dioxide
Principal Display Panel
VALUE SIZE
365 TABLETS
NDC 41250-329-51
Compare to Benadryl®
Allergy ULTRATAB®
active ingredient*
meijer®
allergy
relief
Diphenhydramine HCl | 25 mg
Antihistamine
Relief for: Sneezing, Runny Nose,
Itchy Throat & Itchy, Watery Eyes
365 Tablets
Actual
Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by
Johnson & Johnson Corporation, owner of the registered trademark
Benadryl® Allergy ULTRATAB® . 50844 REV0721D32951
DIST. BY MEIJER
DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544
www.meijer.com
Meijer L-1214-329-51-UPCR REV0721D