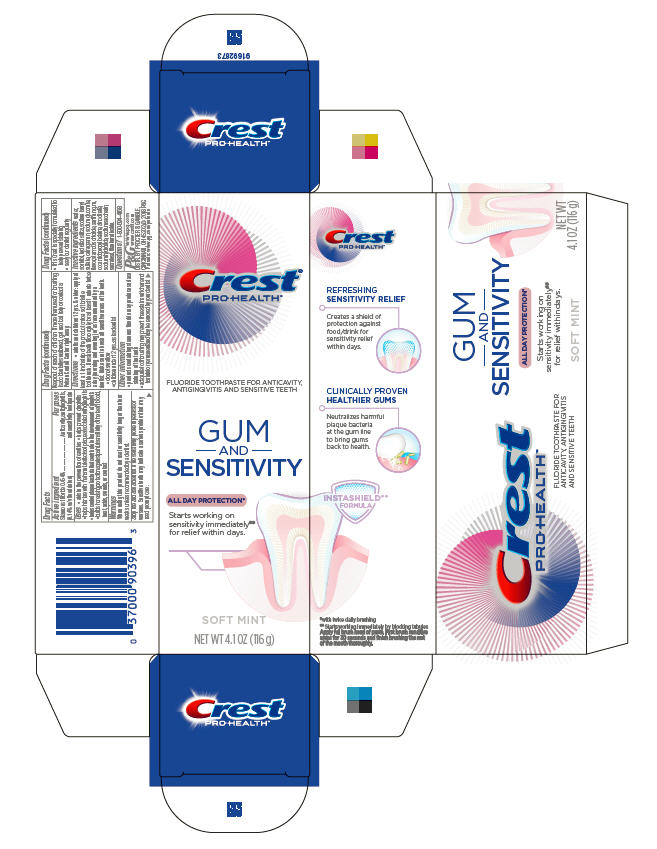
Uses
- aids in the prevention of cavities
- helps prevent gingivitis
- helps interfere with the harmful effects of plaque associated with gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
Warnings
When using this product do not use for sensitivity longer than four weeks unless recommended by a dentist.
Directions
- adults and children 12 yrs. & older: apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist. Make sure to brush all sensitive areas of the teeth.
- do not swallow
- children under 12 yrs.: ask a dentist
Other information
- products containing stannous fluoride may produce surface staining of the teeth
- adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dentist
- this Crest is specially formulated to help prevent staining
- see your dentist regularly