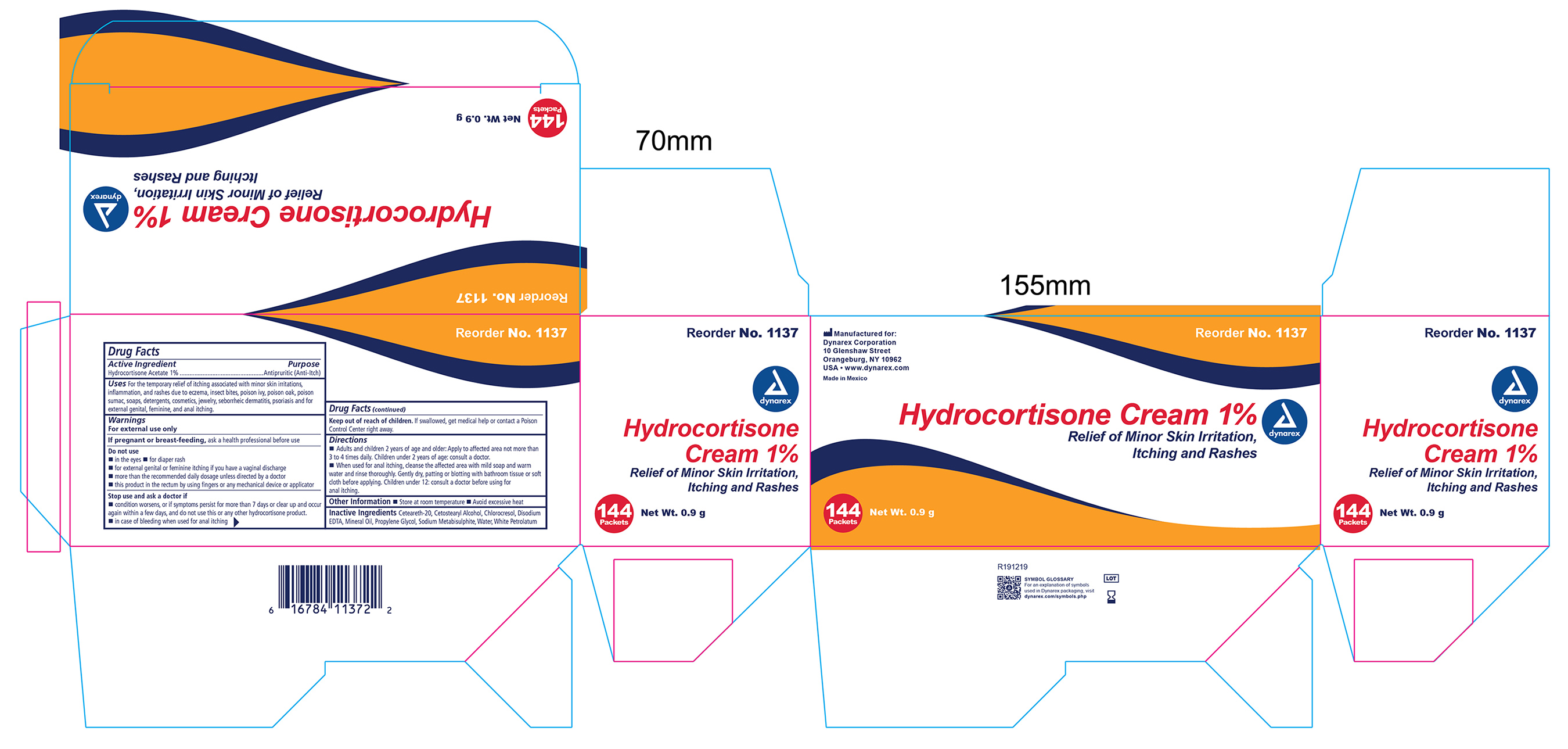
Uses
For temporary relief of itching associated with minor skin irritations, inflammation, and rashes due to eczema, insect bites, poison ivy, poison oak, poison sumac, soaps, detergents, cosmetics, jewelry, seborrheic dermatitis, psoriasis and for external genital, feminine, and anal itching.
Warnings
For external use only
Do not use
- in the yes
- for diaper rash
- for external genital or feminine itching if you have a vaginal discharge
- more than the recommended daily dosage unless directed by a doctor
- this product in the rectumby using fingers or any mechanical device or applicator
Directions
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 time daily. Children under 2 years of age: consult a doctor.
- When used for anal itching, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry, patting or blotting with bathroom tissue or soft cloth before applying. Children under 12: consult a doctor before using for anal itching.