Back Label
Physicians Care
CLEANSE
20 Antiseptic Wipes
20 Alcohol Pads
TREAT
6 Antibiotic Ointment Packets
6 Burn Ointment Packets
10 3" Cotton Tip Applicators
3 Knuckle Bandages
3 Fingertip Bandages
35 3/8” x 1 1/2” Bandages
16 1” x 3” Bandages
200 3/4” x 3” Bandages
1 1/2" Tape
1 Triangular Bandage
4 3" x 3" Sterile Gauze Pads
1 Abdominal Compress
Medicine
3 Acetaminophen Packets (packet of 2)
3 Aspirin Packets (packets of 2)
3 Antacid Packets (packets of 2)
Other
Gloves (2 Pair)
1 Cold Pack
1 First Aid Guide
10 Finger Splints
5 Disposable Thermometers
1 Tweezer
Acme United Corporation
60 Round Hill Road
Fairfield CT 06824
www.acmeunited.com
Designed in the USA | Made in China
CAUTION: This product may contain natural rubber latex which may cause allergic reactions.
This kit contains products that have expiration dates. Please check before use.
BZK Towelette Labeling
Reorder 855
NDC 65517-0004-1
Dukal
BZK TOWELETTE
Contains Benzalkonium Chloride
For External Use Only
Helps Prevent Infection
1 / Pouch
DUKAL CORPORATION
(631) 656-3800
Ronkonkoma, NY 11779 www.dukal.com
Made in China
Drug Facts
Active Ingredients....................Benzalkonium Chloride 0.133% w/v
Purpose................................. First Aid Antiseptic
USE: Antiseptic Cleansing of face, hands and
body without soap and water. Airs dries in seconds
DO NOT USE: in the eyes or apply over large
areas of the body.
STOP USE: If irritation, redness or other symptoms
develop. Consult a doctor if the conditions persists
or gets worse.
CAUTION: Keep out of reach of children. If
swallowed get medical help or contact a Poison
Control Center right away.
DIRECTIONS: Tear open packet, unfold and use
as a washcloth.
INACTIVE INGREDIENTS: Distilled Water
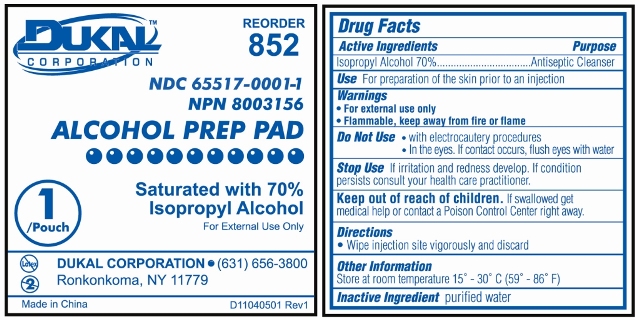
Prep Pad Labeling
Reorder 852
Dukal Corporation
NDC 65517-0001-1
NPN 80003156
ALCOHOL PREP PAD
Saturated with 70% Isopropyl Alcohol
For External Use Only
1 / Pouch
Dukal Corporation
Ronkonkoma, NY 11779
631-656-3800
www.dukal.com
Made in China
Drug Facts
Active Ingredients
Isopropyl Alcohol 70%
Purpose
Antiseptic CleanserUse
For Preparation of Skin prior to an injectionWarnings
- For External Use Only
- Flammable, Keep away from fire or flame
Do Not Use
- with electrocautery procedures
- In the Eyes. If contact occurs, flush eyes with water
Stop Use
If irritation and redness develop. If condition persists, consult your health care practitioner.Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.Directions
Wipe injection site vigorously and discard.Other Information
Store at Room Temperature 15 - 30 C (59 - 86 F)Inactive Ingredient
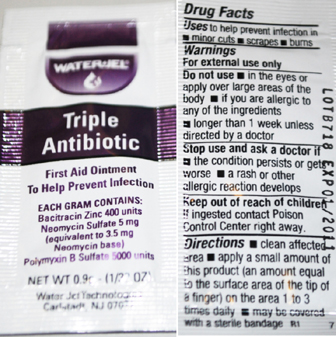
purified waterTriple Antibiotic Labeling
WaterJel
Triple Antibiotic
First Aid Ointment
To Help Prevent Infection
Each Gram contains
Bacitracin Zinc 400 units
Neomycin Sulphate 5 mg
(equivalent to 3.5 mg Neomycin base)
Polymyxin B Sulfate 5000 units
Water-Jel Technologies
Carlstadt, NJ 07072
Drug Facts
Uses to help prevent infection in
minor cuts, scrapes, burns
Warnings
For external use only
Do not use
in the eyes or apply over large areas of the body
If you are allergic to any of the ingredients
longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
the condition persists or gets worse
a rash or other allergic reaction develops
Keep out of reach of children
if ingested contact Poison Control Center right away
Directions
clean affected area apply a small amount of product
(an amount equal to the surface area of the tip of a finger)
on the area 1 to 3 times daily may be covered with a sterile bandage
First Aid Burn Cream Labeling
WaterJel
First Aid Burn Cream
Antiseptic Pain Relief with Aloe
Active Ingredients:
Benzalkonium Chloride 0.13%
Lidocaine HCL 0.5%
Water-Jel Technologies
Carlstadt, NJ 07072
Drug Facts
Purpose
First Aid Antiseptic, External analgesic
Uses
first aid to help prevent infection and for temporary
relief of pain an itching associated with minor cuts,
scrapes, burns
Warnings
For external use only
Do not use
in the eyes
in large quantities over raw or blistered areas or on
deep puncture wounds, animal bites, or serious burns
Keep out of reach of children
if ingested contact Poison Control Center right away
Directions
clean affected area apply a small amount not more
than 3 times daily may be covered with a sterile bandage
Other Information
Store at room temperature
APAP Labeling
Drug Facts
Active Ingredients (In each tablet)...............Purposes
Acetaminophen 325 mg.............................Pain Reliever/Fever Reducer
Uses:
For the temporary relief of minor aches and pains associated with:
• headache • muscular aches • minor arthritis pain
• common cold • toothache • menstrual cramps
For the reduction of fever.
Warnings:
Alcohol Warning: If you consume 3 or more alcoholic drinks every day,
ask your doctor whether you should take acetaminophen or other pain
relievers/fever reducers. Acetaminophen may cause liver damage.
Do not use:
• with any other product containing acetaminophen
• for more than 10 days for pain unless directed by a doctor
• for more than 3 days for fever unless directed by a doctor
Stop Using and Ask a Doctor If:
• symptoms do not improve
• new symptoms occur
• pain or fever persists or gets worse
• redness or swelling is present
Do not exceed recommended dosage. Keep this and all drugs out of the reach of children.
In case of accidental overdose, contact a physician or Poison Control Center immediately.
Prompt medical attention is critical for adults as well as for children even if you do not notice
any signs or symptoms.
If pregnant or breast feeding, ask a health professional before use.
Directions:
Do not use more than directed
Adults and children: (12 years and older)
Take 2 tablets every 4 to 6 hours as needed. Do not take more than 12 tablets in 24 hours,
or as directed by a doctor.
Children under 12 years:
Do not give to children under 12 years of age.
Other Information:
• Store at room temperature
• Tamper-Evident Sealed Packets
• Do Not Use any Opened or Torn Packets
Inactive Ingredients:
Corn starch*, croscarmellose sodium, crospovidone, hypromellose, microcrystalline cellulose,
mineral oil, opadry clear, pharmaceutical glaze, polyethylene glycol, povidone*, pregelatinized
starch*, sodium carboxymethylcellulose, sodium starch glycolate, stearic acid*, talc*, titanium dioxide.
*may contain
Questions or comments? 1-800-634-7680
Alcalak Labeling
Drug Facts
Active Ingredients (In each tablet)....................Purposes
Calcium Carbonate 420 mg ..............................Antacid
Uses:
For the relief of the following symptoms associated with:
• acid indigestion • sour stomach • heartburn • upset stomach
Warnings:
Ask a doctor or health professional before use if you have:
• been taking a prescription drug. Antacids may interact with certain prescription drugs.
• kidney stones
• a calcium-restricted diet
Stop using this product and ask a doctor if:
• symptoms last more than 2 weeks
Do not exceed recommended dosage.
Keep this and all drugs out of the reach of children. If you are pregnant or breast feeding,
ask a health professional before use.
Directions:
• Do not use more than directed
Adults and children: (12 years and older)
Take 2 tablets every 2 or 3 hours as symptoms occur or as directed by a physician. Do not
take more than 19 tablets in a 24 hour period, or use the maximum dosage of this product for
more than 2 weeks, except under the advice and supervision of a physician.
Children under 12 years:
Do not give to children under 12 years of age.
Other Information:
• Phenylketonurics: Contains Phenylalanine 1.5 mg per tablet
• Each tablet contains 168 mg of elemental calcium
• Store at room temperature in a dry place
• Tamper-Evident Sealed Packets
• Do not Use any Opened or Torn Packets
Inactive Ingredients:
aspartame*, carageenan*, croscarmellose sodium*, glycine*, magnesium stearate, mint flavor*,
silica*, sorbitol, spearmint flavor*, stearic acid*, stevix*, xylitol*
*may contain
Questions or comments? 1-800-634-7680
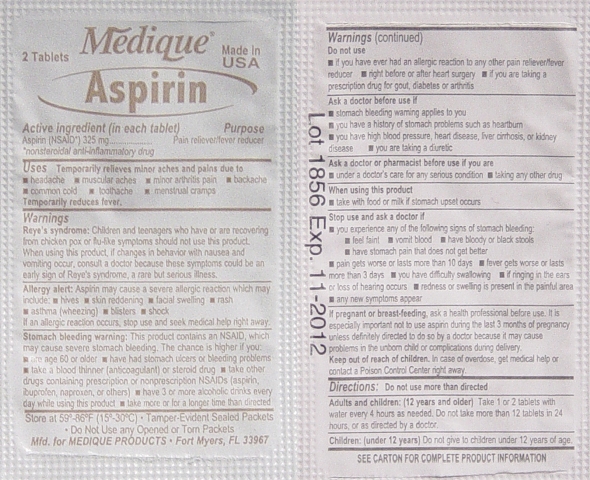
Aspirin Labeling
Drug Facts
Active Ingredients (In each tablet)..................Purposes
Aspirin 325mg (NSAID*) ...............................Pain Reliever / Fever Reducer
*nonsteroidal anti-inflammatory drug
Uses:
Temporarily relieves minor aches and pains due:
• headache • muscular aches • minor arthritis pain • backache
• common cold • toothache • menstrual cramps
Temporarily reduces fever.
Warnings:
Reye's Syndrome: Children and teenagers should not use this medicine for chicken pox,
or flu symptoms before a doctor is consulted about Reye's Syndrome, a rare but serious
illness reported to be associated with aspirin.
Allergy Alert: Aspirin may cause a severe allergic reaction which may include:
• hives • skin reddening • facial swelling • rash • asthma (wheezing) • blisters • shock
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach Bleeding Warning: This product contains a nonsteroidal anti-inflammatory
drug (NSAID), which may cause stomach bleeding.
The chance is higher if you:
• are age 60 or older • have had stomach ulcers or bleeding problems
• take a blood thinning (anticoagulant) or steroid drug
• take other drugs containing an NSAID (aspirin, ibuprofen, naproxen, or others)
• have 3 or more alcohol drinks every day while using this product
• take more or for a longer time than directed
Do not use:
• if you have ever had an allergic reaction to any other pain reliever/ fever reducer.
• right before or after heart surgery
Ask a Doctor before use if you have:
• problems or serious side effects from taking pain relievers or fever reducers
• stomach problems that last or come back, such as heartburn, upset stomach, or
stomach pain • ulcers • bleeding problems • high blood pressure
• heart or kidney disease • taken a diuretic • reached age 60 or older
Ask a Doctor or Pharmacist before use if you are:
• taking any other drug containing and NSAID (prescription or nonprescription)
• taking a blood thinning (anticoagulant) or steroid drug
• under a doctor’s care for any serious condition • taking any other drug
When using this product:
• take with food or milk if stomach upset occurs
• long term continuous use may increase the risk of heart attack or stroke
Stop Using and Ask a Doctor If:
• you feel faint, vomit blood, or have bloody or black stools. These are signs of
stomach bleeding. • pain gets worse or lasts more than 10 days
• fever gets worse or lasts more than 3 days • you have difficulty swallowing
• it feels like the pill is stuck in your throat • you develop heartburn
• stomach pain or upset gets worse or lasts
• redness or swelling is present in the painful area • any new symptoms appear
If pregnant or breast-feeding, ask a health professional before use. It is especially
important not to use aspirin during the last 3 months of pregnancy unless definitely
directed to do so by a doctor because it may cause problems in the unborn child or
complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a
Poison Control Center right away.
Directions:
• Do not take more than directed.
• The smallest effective dose should be used.
• Do not take longer than 10 days, unless directed by a doctor (see Warnings)
• Drink a full glass of water with each dose.
Adults and children: (12 years and older)
Take 1 or 2 tablets with water every 4 hours as needed. Do not take more than 12 tablets
in 24 hours, or as directed by a doctor.
Children under 12 years:
Do not give to children under 12 years of age.
Other Information:
• Read all product information before using
• Store at room temperature
• Avoid excessive heat and humidity
• Tamper evident sealed packets
• Do not use any opened or torn packets
Inactive Ingredients:
Corn starch, croscarmellose sodium*, hypromellose*, microcrystalline cellulose*, mineral oil*,
polyethylene glycol, propylene glycol, silicon dioxide, starch, stearic acid, talc, titanium dioxide*.
*may contain
Questions or comments? 1-800-634-7680