Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help your child get to sleep
Warnings
Do not usein a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if the child has
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
Directions
- do not give more than 6 doses in any 24-hour period
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by a doctor
| Age | Dose |
|---|---|
| children 6 years to under 12 years of age | 5 mL – 10 mL every 4 hours |
| children 4 years to under 6 years of age | 2.5 mL – 5 mL every 4 hours |
| children under 4 years of age | do not use |
Inactive ingredients
ammonium glycyrrhizate, anhydrous citric acid, edetate disodium, flavors, glycerin (soy), propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, xanthan gum
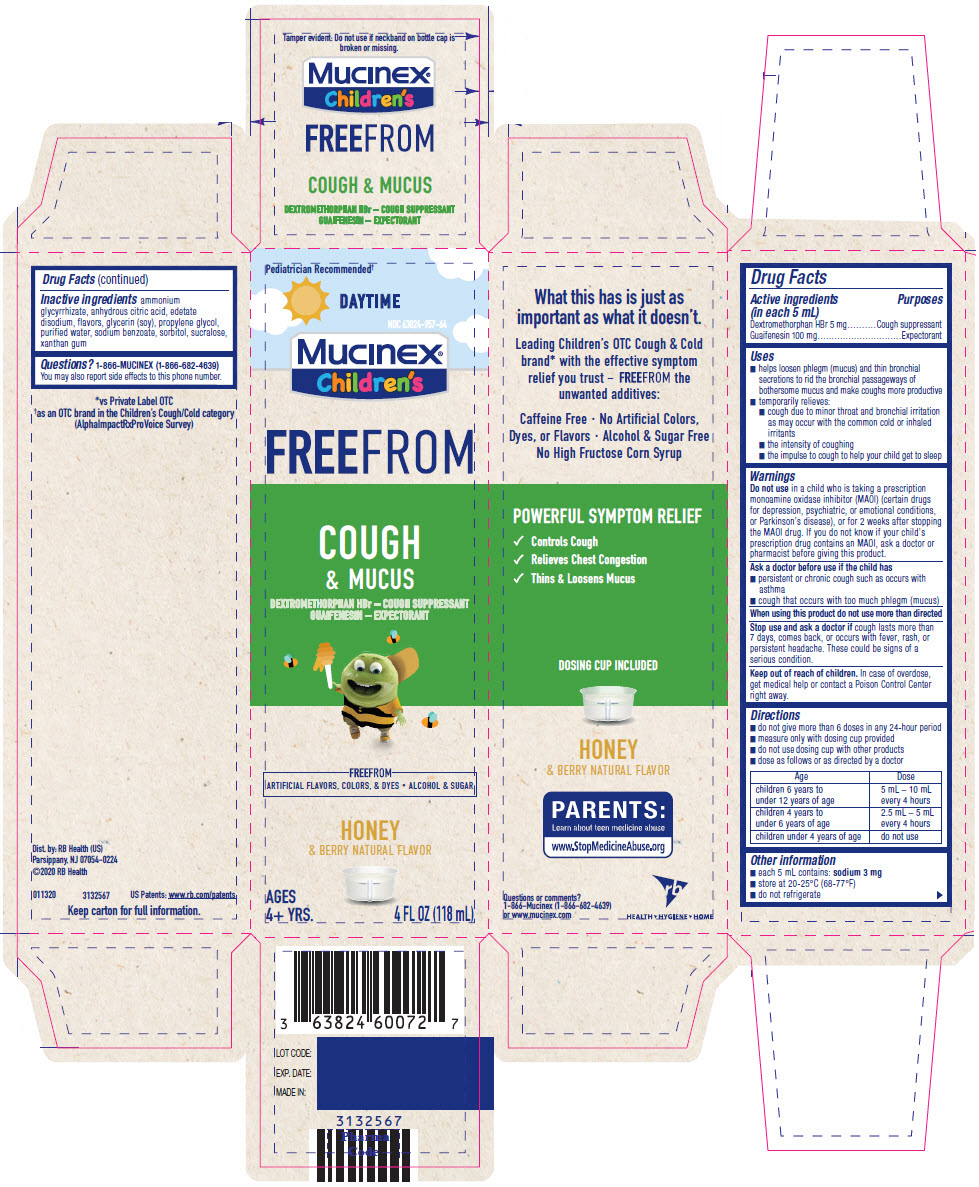
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
Pediatrician Recommended†
DAYTIME
NDC 63824-957-64
Mucinex®
Children's
FREEFROM
COUGH
& MUCUS
DEXTROMETHORPHAN HBr – COUGH SUPPRESSANT
GUAIFENESIN – EXPECTORANT
FREEFROM
ARTIFICIAL FLAVORS, COLORS, & DYES • ALCOHOL & SUGAR
HONEY
& BERRY NATURAL FLAVOR
AGES
4+ YRS.
4 FL OZ (118 mL)