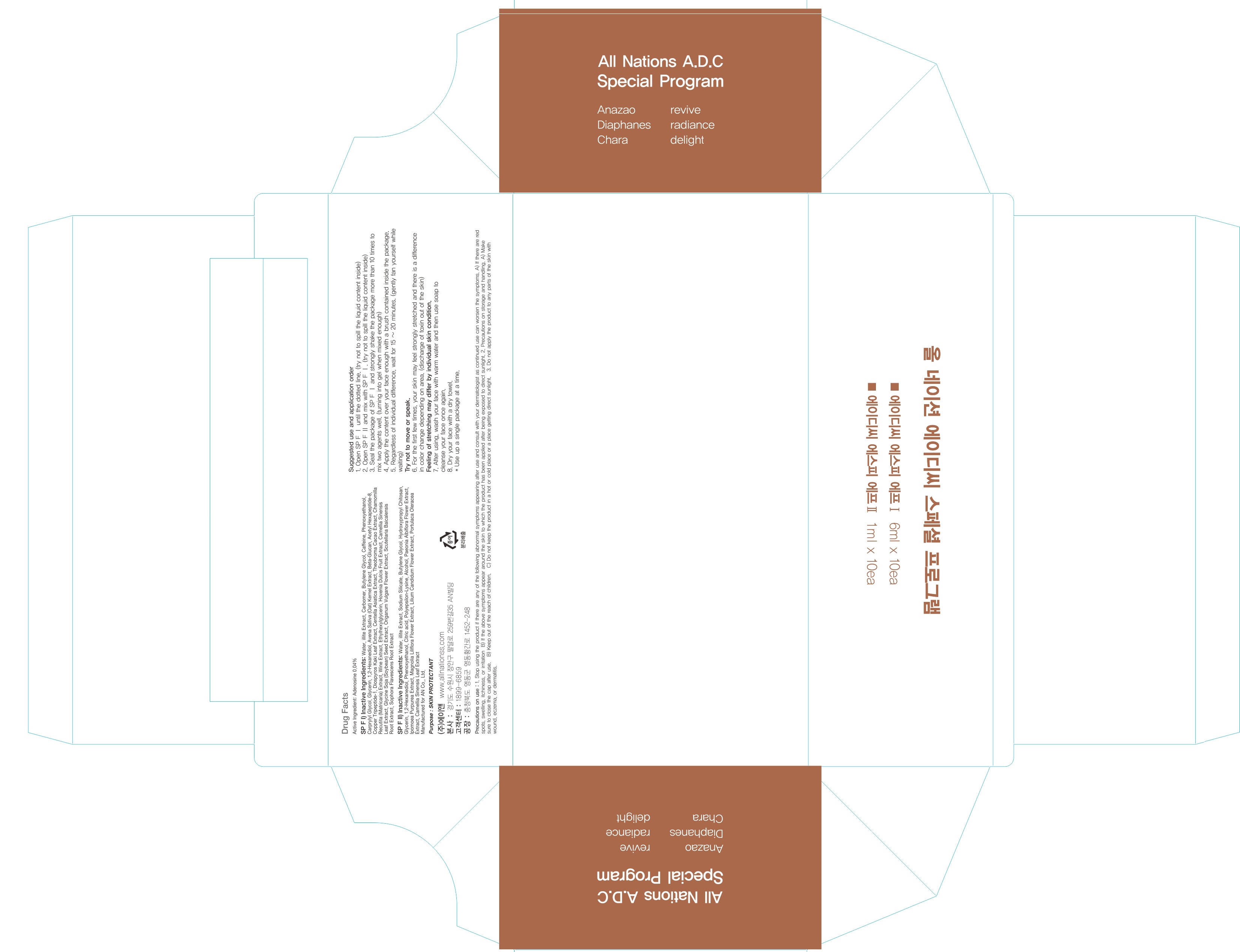
INACTIVE INGREDIENT
Inactive ingredients:
SP F I) Water, illite Extract, Carbomer, Butylene Glycol, Caffeine, Phenoxyethanol, Carprylyl Glycol, Glycerin, 1,2-Hexanediol, Avena Sativa (Oat) Kernel Extract, Beta-Glucan, Acetyl Hexapeptide-8, Copper Tripeptide-1, Diospyros Kaki Leaf Extract, Centella Asiatica Extract, Theobroma Cacao Extract, Chamomilla Recutita (Matricaria) Extract, Wine Extract, Ethylhexylglycerin, Hovenia Dulcis Fruit Extract, Camellia Sinensis Leaf Extract, Glycine Soja (Soybean) Seed Extract, Origanum Vulgare Flower Extract, Scutellaria Baicalensis Root Extract, Sophora Flavescens Root Extract
SP F II) Water, illite Extract, Sodium Silicate, Butylene Glycol, Hydroxypropyl Chitosan, Glycerin, 1,2-Hexanediol, Phenoxyethanol, Citric acid, Polyepsilon-Lysine, Alcohol, Paeonia Albiflora Flower Extract, Ipomoea Purpurea Extract, Magnolia Liliflora Flower Extract, Lilium Candidum Flower Extract, Portulaca Oleracea Extract, Camellia Sinensis Leaf Extract
Volume) SP F I: 6mL, SP F II: 1mL
Precautions on use
Precautions on use: 1. Stop using the product if there are any of the following abnormal symptoms appearing after use and consult with your dermatologist as continued use can worsen the symptoms. A) If there are red spots, swelling, itchiness, or irritation B) If the above symptoms appear around the skin to which the product has been applied after being exposed to direct sunlight. 2. Precautions on storage and handling. A) Make sure to close the cap after use. B) Keep out of the reach of children. C) Do not keep the product in a hot or cold place or a place getting direct sunlight. 3. Do not apply the product to any parts of the skin with wound, eczema, or dermatitis.
INDICATIONS & USAGE
Suggested use and application order:
1. Open SP F Ⅰ until the dotted line. (try not to spill the liquid content inside) 2. Open SP F Ⅱ and mix with SP F Ⅰ. (try not to spill the liquid content inside) 3. Seal the package of SP F Ⅰ and strongly shake the package more than 10 times to mix two agents well. (turning into gel when mixed enough) 4. Apply the content over your face enough with a brush contained inside the package. 5. Regardless of individual difference, wait for 15 ~ 20 minutes. (gently fan yourself while waiting) Try not to move or speak. 6. For the first few times, your skin may feel strongly stretched and there is a difference in color change depending on area. (discharge of toxin out of the skin) Feeling of stretching may differ by individual skin condition. 7. After using wash your face with warm water and then use soap to cleanse your face once again. 8. Dry your face with a dry towel.
*Use up a single package at a time.
DOSAGE & ADMINISTRATION
Suggested use and application order:
1. Open SP F Ⅰ until the dotted line. (try not to spill the liquid content inside) 2. Open SP F Ⅱ and mix with SP F Ⅰ. (try not to spill the liquid content inside) 3. Seal the package of SP F Ⅰ and strongly shake the package more than 10 times to mix two agents well. (turning into gel when mixed enough) 4. Apply the content over your face enough with a brush contained inside the package. 5. Regardless of individual difference, wait for 15 ~ 20 minutes. (gently fan yourself while waiting) Try not to move or speak. 6. For the first few times, your skin may feel strongly stretched and there is a difference in color change depending on area. (discharge of toxin out of the skin) Feeling of stretching may differ by individual skin condition. 7. After using wash your face with warm water and then use soap to cleanse your face once again. 8. Dry your face with a dry towel.
*Use up a single package at a time.