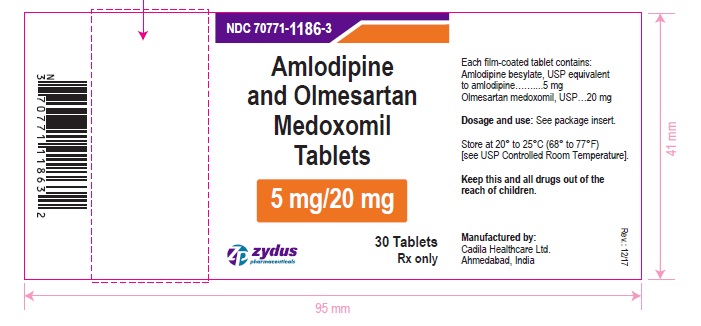
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Amlodipine and olmesartan medoxomil tablets, 5/20 mg
Rx only
30 tablets
Amlodipine and olmesartan medoxomil tablets, 10/20 mg
Rx only
30 tablets

Amlodipine and olmesartan medoxomil tablets, 5/40 mg
Rx only
30 tablets

Amlodipine and olmesartan medoxomil tablets, 10/40 mg
Rx only
30 tablets
