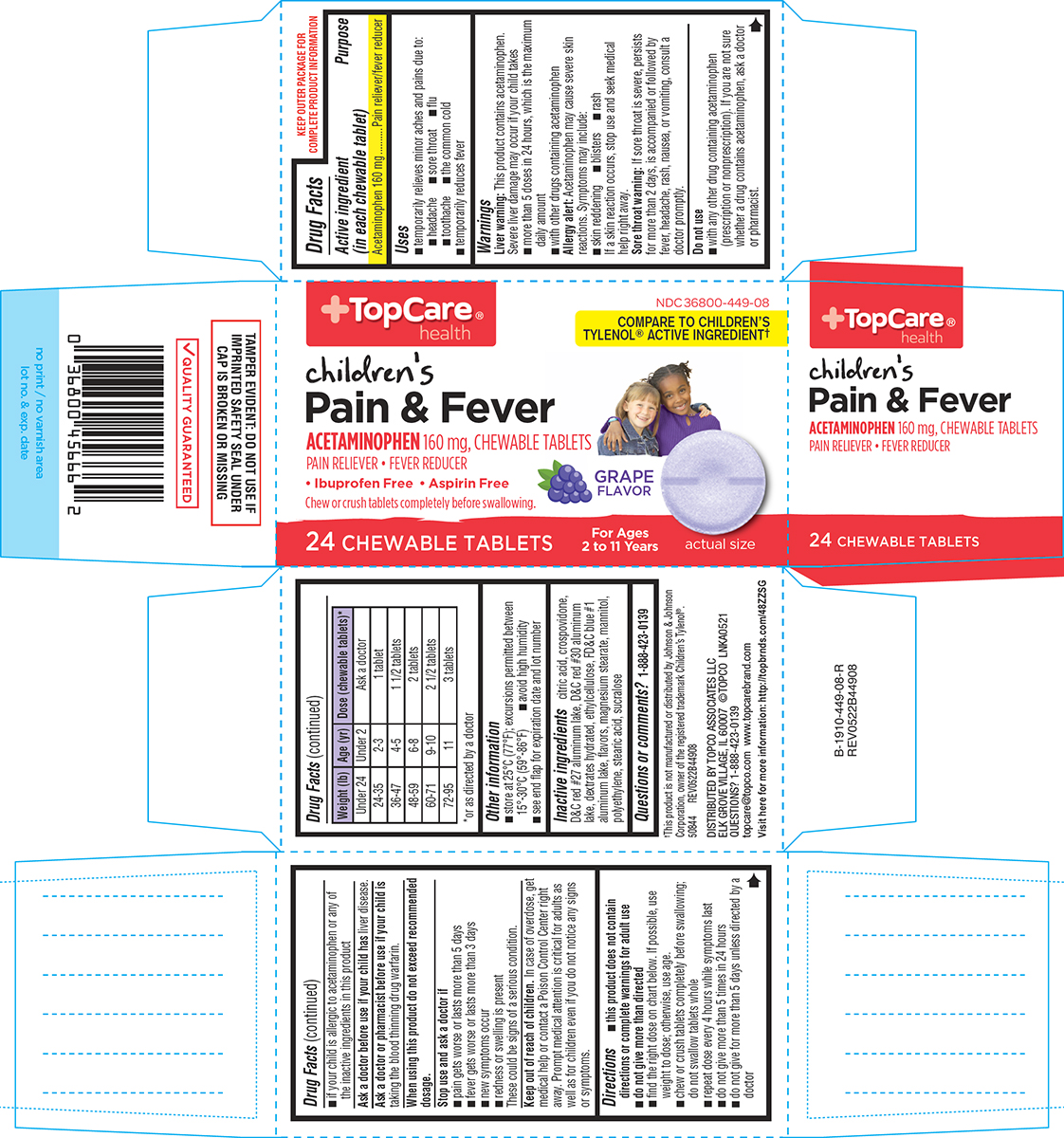
Uses
- temporarily relieves minor aches and pains due to:
- headache
- sore throat
- flu
- toothache
- the common cold
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
| Weight (lb) | Age (yr) | Dose (chewable tablets)*
|
| Under 24 | Under 2 | Ask a doctor |
| 24-35 | 2-3 | 1 tablet |
| 36-47 | 4-5 | 1 1/2 tablets |
| 48-59 | 6-8 | 2 tablets |
| 60-71 | 9-10 | 2 1/2 tablets |
| 72-95 | 11 | 3 tablets |
*or as directed by a doctor
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- avoid high humidity
- see end flap for expiration date and lot number
Inactive ingredients
citric acid, crospovidone, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates hydrated, ethylcellulose, FD&C blue #1 aluminum lake, flavors, magnesium stearate, mannitol, polyethylene, stearic acid, sucralose
Principal Display Panel
+TopCare®
health
NDC 36800-449-08
COMPARE TO CHILDREN’S
TYLENOL® ACTIVE INGREDIENT†
children's
Pain & Fever
ACETAMINOPHEN 160 mg, CHEWABLE TABLETS
PAIN RELIEVER • FEVER REDUCER
• Ibuprofen Free • Aspirin Free
Chew or crush tablets completely before swallowing.
GRAPE
FLAVOR
24 CHEWABLE TABLETS
For Ages
2 to 11 Years
actual size
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER
CAP IS BROKEN OR MISSING
†This product is not manufactured or distributed by Johnson & Johnson
Corporation, owner of the registered trademark Children’s Tylenol®.
50844 REV0522B44908
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007 ©TOPCO LNKA0521
QUESTIONS? 1-888-423-0139
topcare@topco.com www.topcarebrand.com
Visit here for more information: http://topbrnds.com/48ZZSG
TopCare 44-449