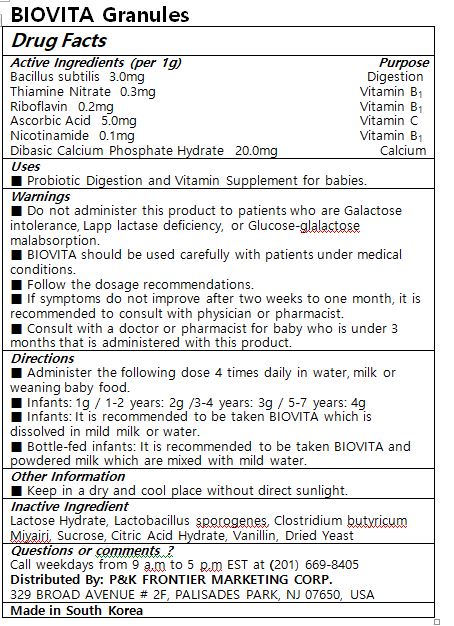
Active ingredients (1g contains)
Bacillus subtilis 3.0 mg
Thiamine Nitrate 0.3 mg
Riboflavin 0.2 mg
Ascorbic Acid 5.0 mg
Nicotinamide 0.1 mg
Dibasic Calcium Phosphate Hydrate 20.0 mg
Administer the following dose 4 times daily in water, milk or weaning baby food.
Infants: 1g / 1-2 years: 2g /3-4 years: 3g / 5-7 years: 4g
Infants: It is recommended to be taken BIOVITA which is dissolved in mild milk or water.
Bottle-fed infants: It is recommended to be taken BIOVITA and powdered milk which are mixed with mild water.
Do not administer this product to patients who are Galactose intolerance, Lapp lactase deficiency, or Glucose-glalactose malabsorption.
BIOVITA should be used carefully with patients under medical conditions.
Follow the dosage recommendations.
If symptoms do not improve after two weeks to one month, it is recommended to consult with physician or pharmacist.
Consult with a doctor or pharmacist for baby who is under 3 months that is administered with this product.
Keep in a dry and cool place without direct sunlight.