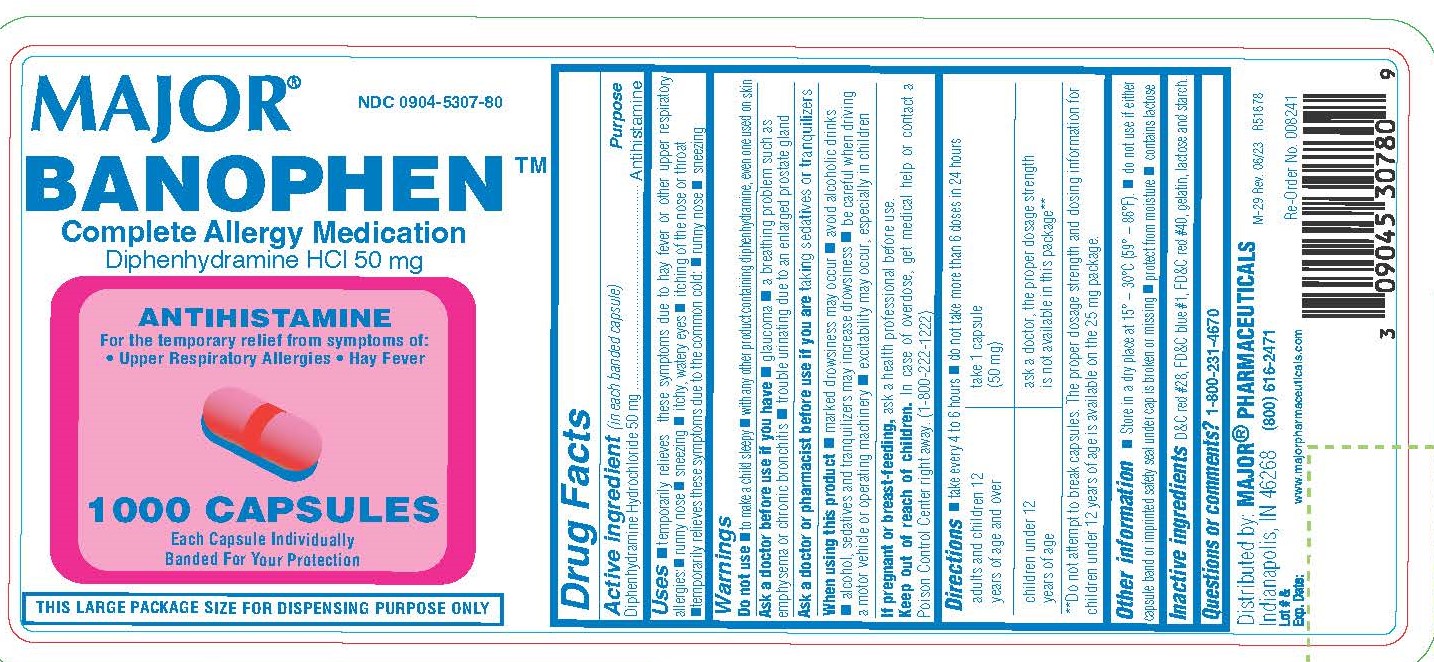
Use
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat and nose
- Temporarily relieves these symptoms due to the common cold
- runny nose
- sneezing
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Directions
- Take every 4-6 hours
- Do not take more than 6 doses in 24 hours
| adults and children 12 years of age and over | Take 1 capsule (50 mg) |
| children under 12 years of age | ask a doctor, the proper dosage strength is not available in this package** |
| **Do not attempt to break capsules. The proper dosage strength and dosing information for children under 12 years of age is available on the 25 mg package. | |
Other Information
- Store in a dry place at 15° – 30°C (59° – 86°F).
- Do not use if either capsule band or imprinted safety seal under cap is broken or missing
- Protect from moisture
- Contains lactose
Distributed by: MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
(800) 616-2471
www.majorpharmaceuticals.com
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Major
NDC 0904-5307-80
BENOPHEN
Complete Allergy Medication
Diphenhydramine HCl 50 mg
ANTIHISTAMINE
For the temporary relief from symptoms of:
• Upper Respiratory Allergies • Hay Fever
1000 CAPSULES
Each Capsule Individually Banded For Your Protection
THIS LARGE PACKAGE SIZE FOR DISPENSING PURPOSE ONLY