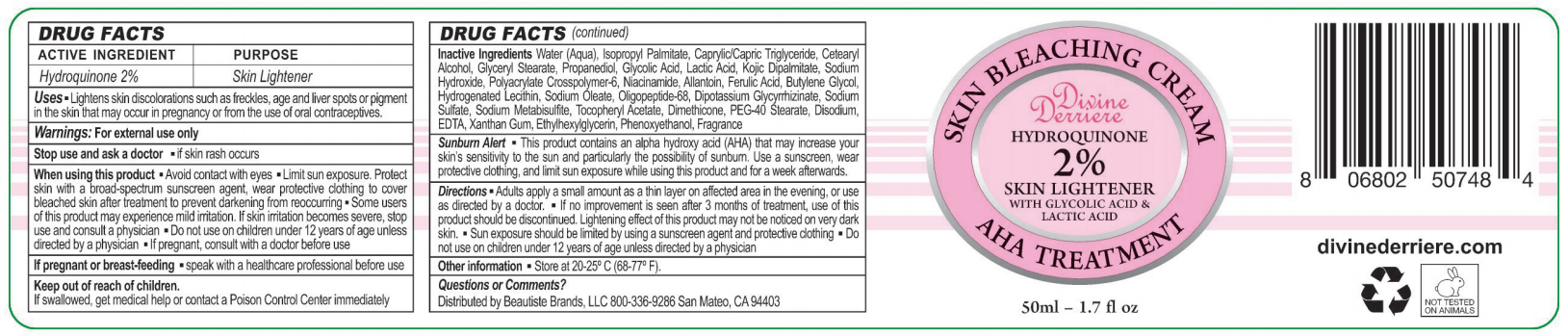
Uses
- Lightens skin discoloration sich as freckles, age and liver spots or pigment in the skin that may occur in pregnancy or from the use of oral contraceptions
Warnings: for external use only
When using this product
- Avoid contact with eyes
- Limit sun exposure. Protect skin with a Broad-Spectrum sunscreen agent, wear protective clothing to cover bleached skin after treatment to prevent darkening from reoccuring
- Some users of this product may experience mild irritation. If skin irritation becomes severe, stop use and consult a physician
- Do not use on children under 12 years of age unless directed by a physician
- If product consult with a doctor before use
- If pregnant or breast-feeding
- Speak with a healthcare professional before use
Sunburn alert
- This product contains an alpha-hydroxy acid (AHA) that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product and for a week afterwards
Inactive Ingredients Water(Aqua), Isopropyl Palmitate, Caprylic/capric Triglyceride, Cetearyl Alcohol, Glyceryl Stearate, Propanediol, Glycolic Acid, Lactic Acid, Kojic Dipalmitate, Sodium Hydroxide, Polyacrylate Crosspolymer-6, Niacinamide, Allantoin, Ferulic Acid, Butylene Glycol, hydrogenated Lecithin, Sodium Oleate, Oligopeptide-68, Dipotassium Glycrrhizate, Sodium Sulfate, Sodium Metabisulfite, Tocopheryl Acetate, Dimethicone, PEG-40 Stearate, Disodium EDTA, Xanthan Gum, Ethylhexylglycerin, Phenoxyethanol, Frangrance.