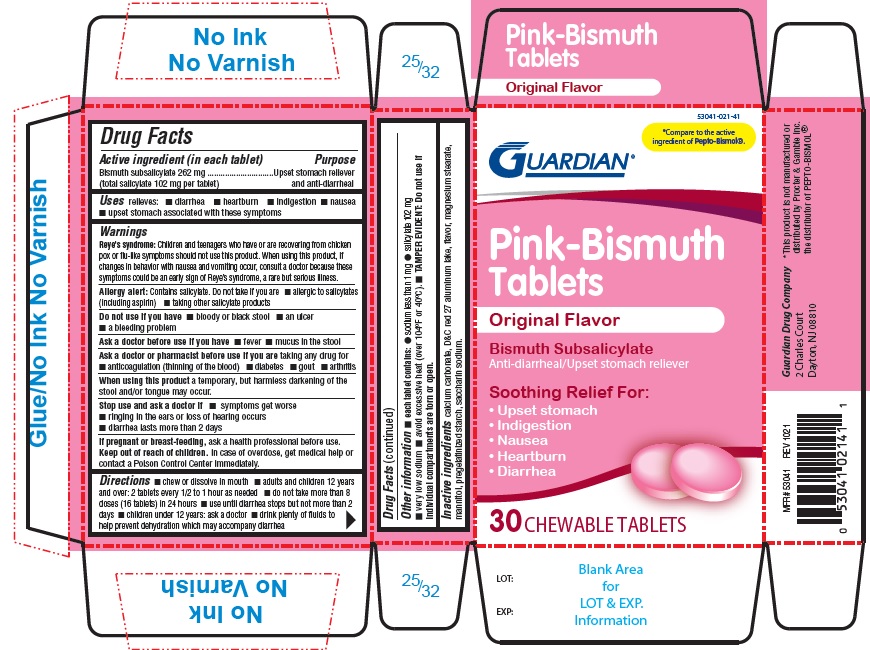
WARNINGS
Reye's Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products
ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU ARE
taking any drug for
- anticoagulation (thinning of the blood)
- diabetes
- gout
- arthritis
STOP USE AND ASK DOCTOR IF
- symptoms get worse
- ringing in the ears or loss of hearing occurs
- diarrhea lasts more than 2 days
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center immediately.
DIRECTIONS
- chew or dissolve in mouth
- adults and children 12 years and over: 2 tablets every 1/2 to 1 hour as needed
- do not take more than 8 doses (16 tablets) in 24 hours
- use until diarrhea stops but not more than 2 days
- children under 12 years: ask a doctor
- drink plenty of fluids to help prevent dehydration caused diarrhea
OTHER INFORMATION
- each tablet contains:
- sodium less than 1 mg
- salicylate 102 mg
- very low sodium
- avoid excessive heat (over 104oF or 40oC)
- TAMPER EVIDENT: Do not use if individual compartments are torn on open.