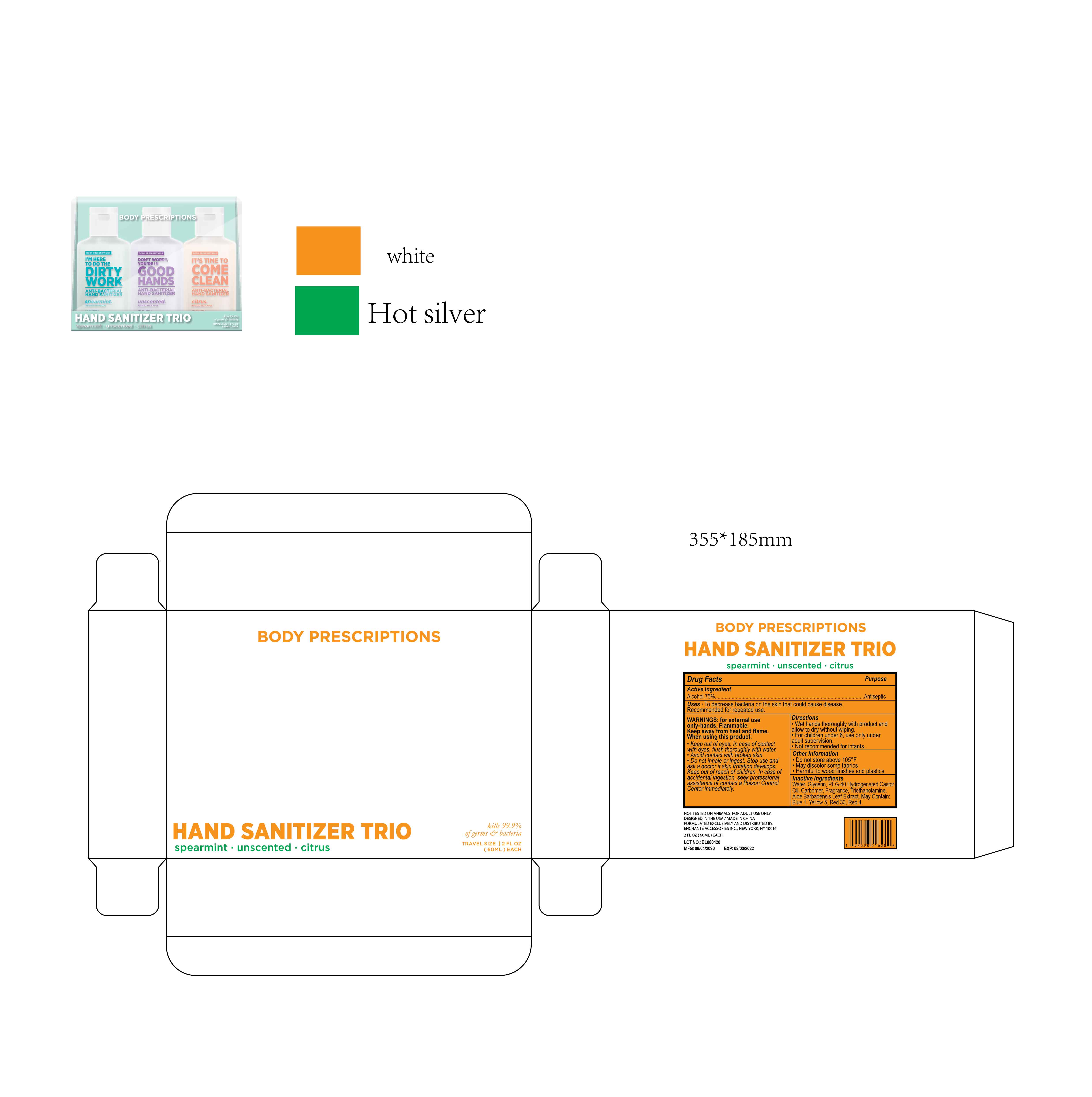
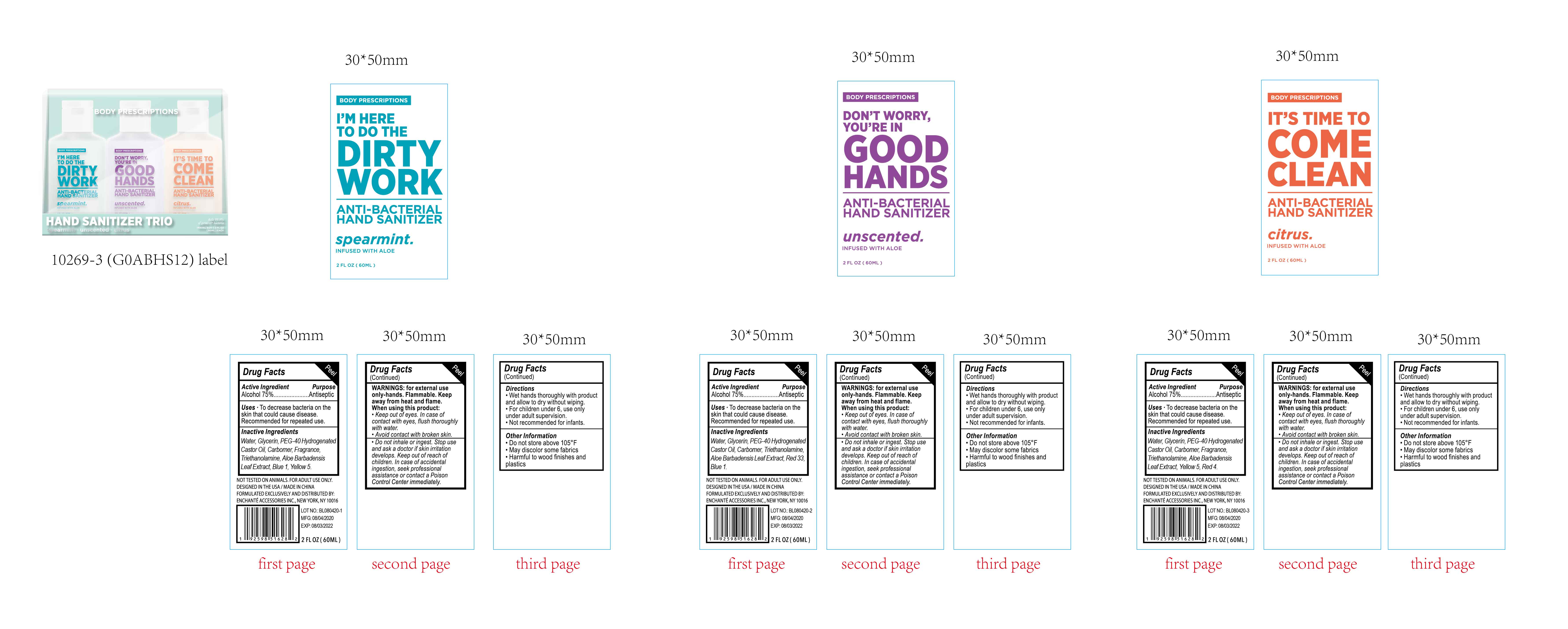
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER Spearmint
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER Spearmint
WARNINGS
For external use only, Hands
Flammable. Keep away from heat and flame
When using this product, keep out of eyes.
In case of contact with eyes, flush thoroughly with water
Avoid contact with broken skin
Do not inhale or ingest
Directions
Wet hands thoroughly with product and allow to dry without wiping
For children under 6, use only under adult supervision
Not recommended for infants
Other information
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics
Inactive ingredients
Water, Glycerin, PEG-40 Hydrogenated Castor Oil, Carbomer, Triethanolamine, Aloe Barbadensis Leaf Extract, Blue 1, Yellow 5
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER Unsented
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER Unscented
Warnings
For external use only, Hands
Flammable. Keep away from heat and flame
When using this product, keep out of eyes.
In case of contact with eyes, flush thoroughly with water
Avoid contact with broken skin
Do not inhale or ingest
Keep out of reach of children
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a poison control center immediately
Directions
Wet hands thoroughly with product and allow to dry without wiping
For children under 6, use only under adult supervision
Not recommended for infants
Other Information
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics
Inactive Ingredients
Water, Glycerin, PEG-40 Hydrogenated Castor Oil, Carbomer, Triethanolamine, Aloe Barbadensis Leaf Extract, Red 33, Blue 1
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER Citrus
BODY PRESCRIPTIONS ANTIBACTERIAL HAND SANITIZER Citrus
Warnings
For external use only, Hands
Flammable. Keep away from heat and flame
When using this product, keep out of eyes.
In case of contact with eyes, flush thoroughly with water
Avoid contact with broken skin
Do not inhale or ingest
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a poison control center immediately
Directions
Wet hands thoroughly with product and allow to dry without wiping
For children under 6, use only under adult supervision
Not recommended for infants
Other Information
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics