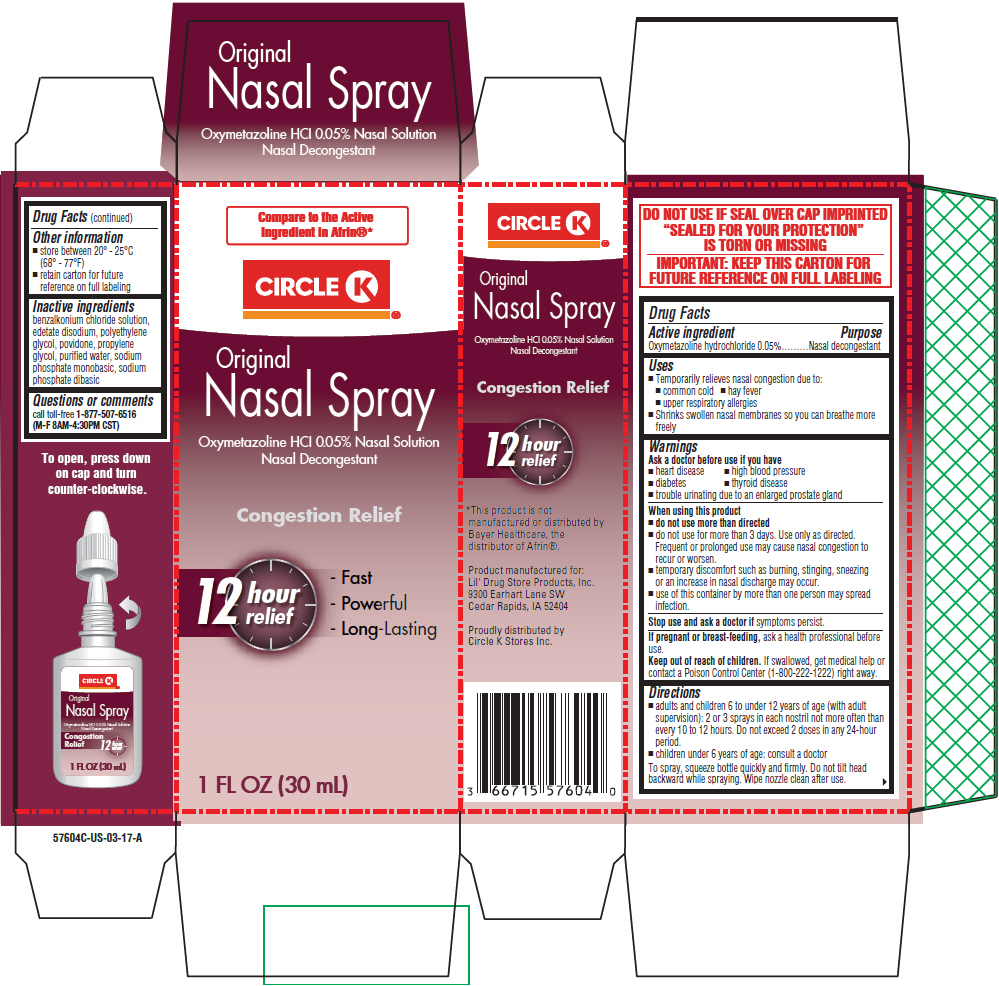
Uses
- Temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- Shrinks swollen nasal membranes so you can breathe more freely
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur.
- use of this container by more than one person may spread infection.
Directions
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- children under 6 years of age: consult a doctor
To spray, squeeze bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use.
Other information
- store between 20° - 25°C (68° - 77°F)
- retain carton for future reference on full labeling
Inactive ingredients
benzalkonium chloride solution, edetate disodium, polyethylene glycol, povidone, propylene glycol, purified water, sodium phosphate monobasic, sodium phosphate dibasic