Warnings
For external use only
When using this product
- avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- limit use to the face and neck
Directions
- wet face
- dispense product into hands, lather and massage gently onto face and neck, avoiding the delicate eye area
- rinse thoroughly with warm water and pat dry
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
water, sodium cocoyl isethionate, cetearyl alcohol, glycerin, sodium laureth sulfate, sodium cocoamphoacetate, cocamidopropyl betaine, sodium hydroxide, acrylates/C10-30 alkyl acrylate crosspolymer, fragrance, disodium EDTA, lavandula stoechas extract, helichrysum italicum extract, cistus monspeliensis extract
Warnings
For external use only
When using this product
- avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- limit use to the face and neck
Directions
- clean the skin thoroughly before applying this product
- wipe a pad over face and neck to cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Other information
- do not flush in toilet
- keep jar lid tightly closed
- store in a cool dry place but do not freeze
Inactive ingredients
water, alcohol denat., glycerin, isoceteth-20, hydrogenated starch hydrolysate, sodium hydroxide, fragrance, lavandula stoechas extract, helichrysum italicum extract, cistus monspeliensis extract, disodium EDTA
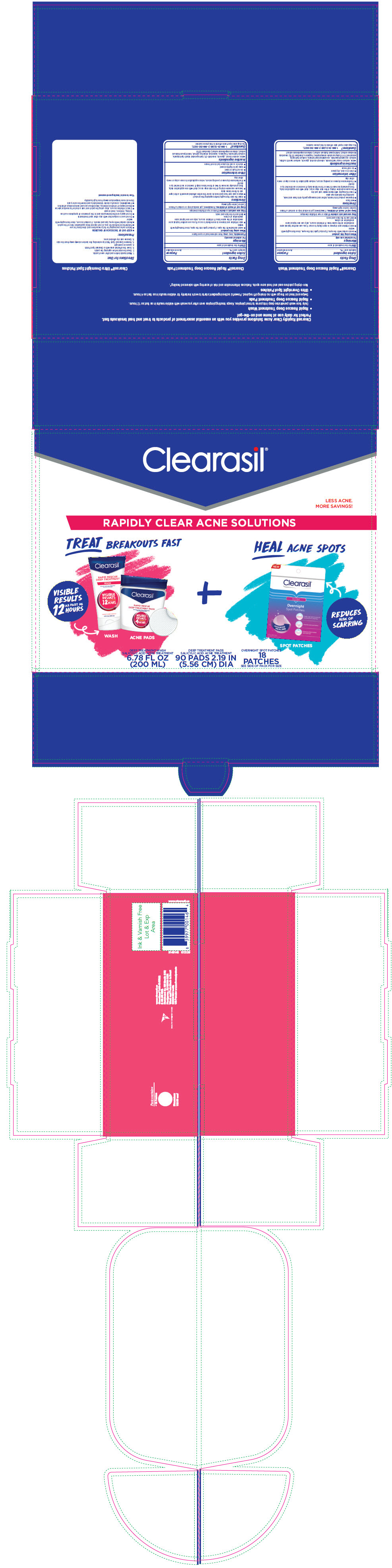
PRINCIPAL DISPLAY PANEL - Kit Carton
Clearasil ®
LESS ACNE.
MORE SAVINGS!
RAPIDLY CLEAR ACNE SOLUTIONS
TREAT BREAKOUTS FAST
VISIBILE
RESULTS
AS FAST AS
12
HOURS
WASH
ACNE PADS
+
HEAL ACNE SPOTS
REDUCES
RISK OF
SCARRING
SPOT PATCHES
DEEP TREATMENT WASH
SALICYLIC ACID ACNE TREATMENT
6.78 FL OZ
(200 ML)
DEEP TREATMENT PADS
SALICYLIC ACID ACNE TREATMENT
90 PADS 2.19 IN
(5.56 CM) DIA
OVERNIGHT SPOT PATCHES
18
PATCHES
SEE SIDE OF PACK FOR SIZE