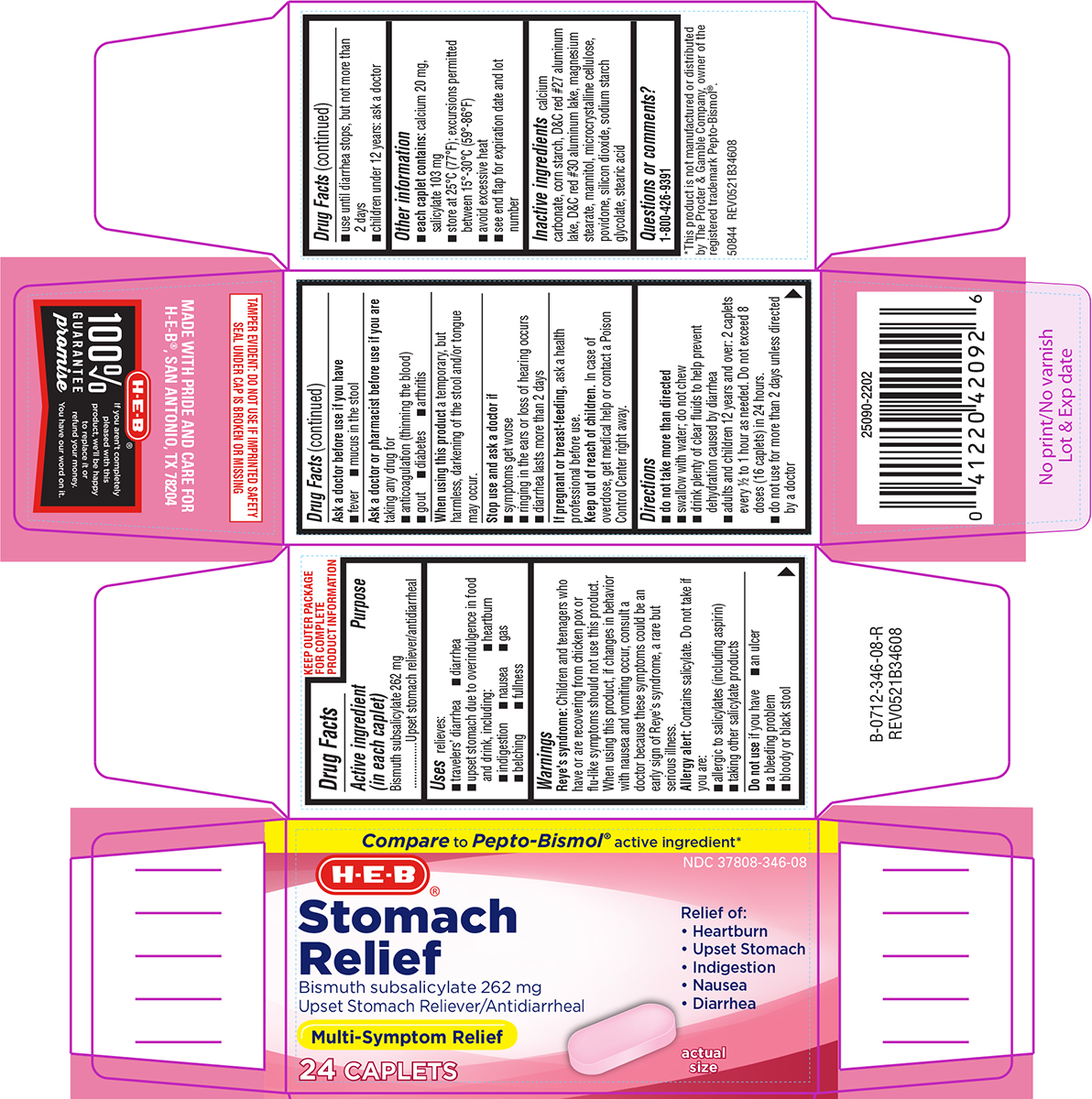
Uses
relieves:
- travelers' diarrhea
- diarrhea
- upset stomach due to overindulgence in food and drink, including:
- belching
- indigestion
- gas
- fullness
- heartburn
- nausea
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are:
- allergic to salicylates (including aspirin)
- taking other salicylate products
Ask a doctor or pharmacist before use if you are
taking any drug for
- anticoagulation (thinning the blood)
- gout
- diabetes
- arthritis
Directions
- do not take more than directed
- swallow with water; do not chew
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- adults and children 12 years and over: 2 caplets every ½ to 1 hour as needed. Do not exceed 8 doses (16 caplets) in 24 hours.
- do not use for more than 2 days unless directed by a doctor
- use until diarrhea stops, but not more than 2 days
- children under 12 years: ask a doctor
Other information
- each caplet contains: calcium 20 mg, salicylate 103 mg
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- avoid excessive heat
- see end flap for expiration date and lot number
Inactive ingredients
calcium carbonate, corn starch, D&C red #27 aluminum lake, D&C red #30 aluminum lake, magnesium stearate, mannitol, microcrystalline cellulose, povidone, silicon dioxide, sodium starch glycolate, stearic acid
Principal display panel
Compare to Pepto-Bismol® active ingredient*
NDC 37808-346-08
H-E-B®
Stomach
Relief
Bismuth subsalicylate 262 mg
Upset Stomach Reliever/Antidiarrheal
Relief of:
• Heartburn
• Upset Stomach
• Indigestion
• Nausea
• Diarrhea
Multi-Symptom Relief
24 CAPLETS
actual
size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed
by The Procter & Gamble Company, owner of the
registered trademark Pepto-Bismol®.
50844 REV0521B34608
MADE WITH PRIDE AND CARE FOR
H-E-B®, SAN ANTONIO, TX 78204
H-E-B®
100%
GUARANTEE
promise
If you aren't completely
pleased with this
product, we'll be happy
to replace it or
refund your money.
You have our word on it.
HEB 44-346