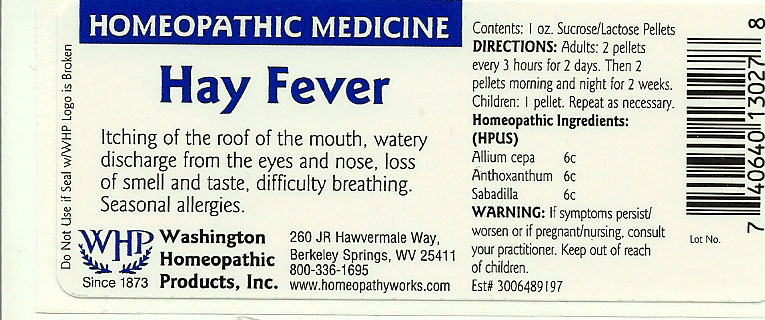
HAY FEVER- onion - anthoxanthum odoratum - schoenocaulon officinale seed pellet
Washington Homeopathic Products
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS
ALLIUM CEPA 6C
ANTHOXANTHUM 6C
SABADILLA 6C
USES
To relieve the symptoms of itching of the roof of the mouth, watery discharge from the eyes and nose, loss of smell and taste, difficulty breathing. Seasonal Allergies.
KEEP OUT OF REACH OF CHILDREN
Keep this and all medicines out of reach of children.
INDICATIONS
Indications:
ALLIUM CEPA Head cold
ANTHOXANTHUM Hay fever
SABADILLA Hay fever
STOP USE AND ASK DOCTOR
If symptoms persist/worsen or if pregnant/nursing, stop use and consult your practitioner.
DIRECTIONS
Adults 2 pellets every 3 hours for 2 days. Then 2 pellets morning and night for 2 weeks.
Children: 1 pellet. Repeat as necessary.
INACTIVE INGREDIENTS
Sucrose/Lactose
PRINCIPAL DISPLAY PANEL
 Enter section text here
Enter section text here
 Enter section text here
Enter section text here