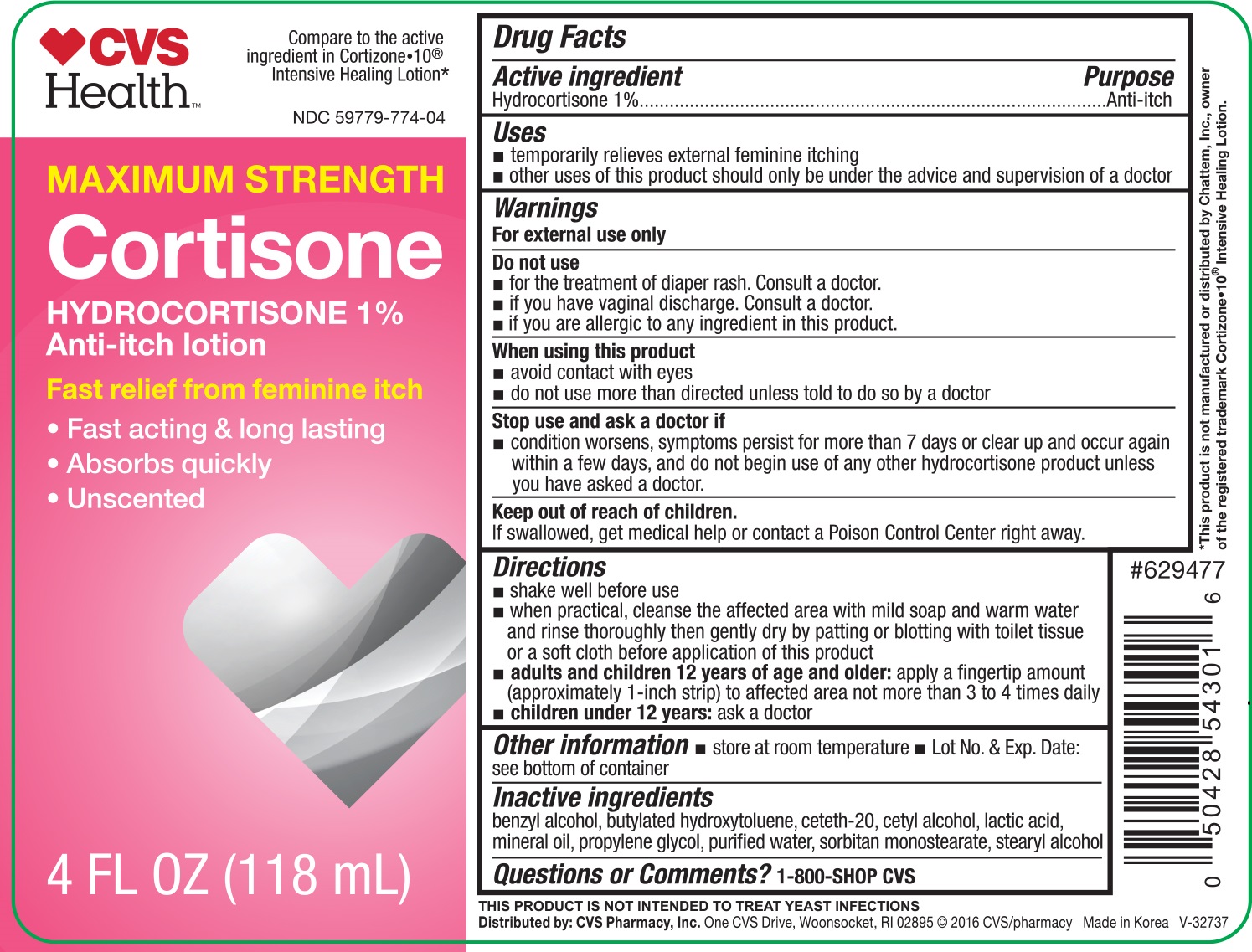
Active ingredient Purpose
Hydrocortisone 1%.................................................. Anti-itch
Uses
- temporarily relieves external feminine itching
- other uses of this product should only be used under the advice and supervision of a doctor
Do not use
- for the treatment of diaper rash. Consult a doctor.
- if you have vaginal discharge. Consult a doctor.
- if you are allergic to any ingredient in this product.
When using this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor
Stop use and ask a doctor if
- condition worsens, symptoms persist for more than 7 days or clear up and occur again within a few days, and do not begin use of any other hydrocortisone product unless you have asked a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake well before use
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly then gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product
- adults and children 12 years of age and older: apply a fingertip amount (approxametly 1-inch strip) to affected area not more than 3 to 4 times daily
- children under 12 years: ask a doctor