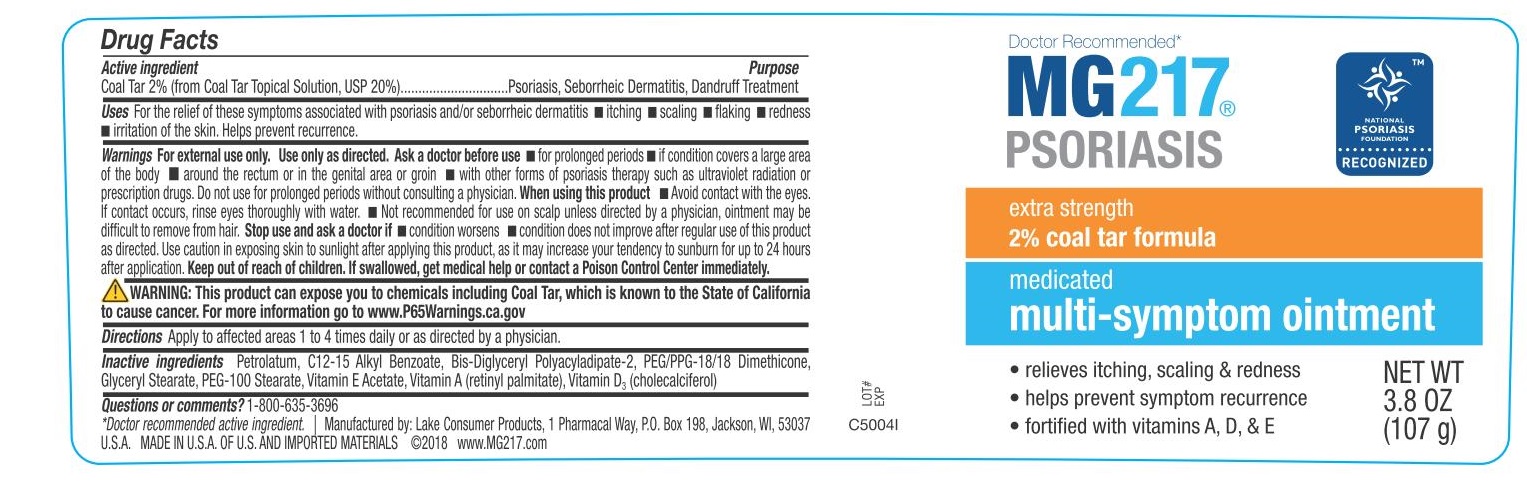
Uses For the relief of these symptoms associated with psoriasis and/or seborrheic dermatitis ■ itching ■ scaling
■ flaking ■ redness ■ irritation of the skin. Helps prevent recurrence.
Warnings
For external use only. Use only as directed.
Ask a doctor before use ■ for prolonged periods ■ if condition covers a large area of the body ■ around the rectum or in the genital area or groin ■ with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs ■ do not use for prolonged periods without consulting a physician.
When using this product ■ Avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water. ■ Not recommended for use on scalp unless directed by a physician, ointment may be difficult to remove from hair.
Stop use and ask a doctor if ■ condition worsens ■ condition does not improve after regular use of this product as directed. Use caution in exposing skin to sunlight after applying this product, as it may increase your tendency to sunburn for up to 24 hours after application.