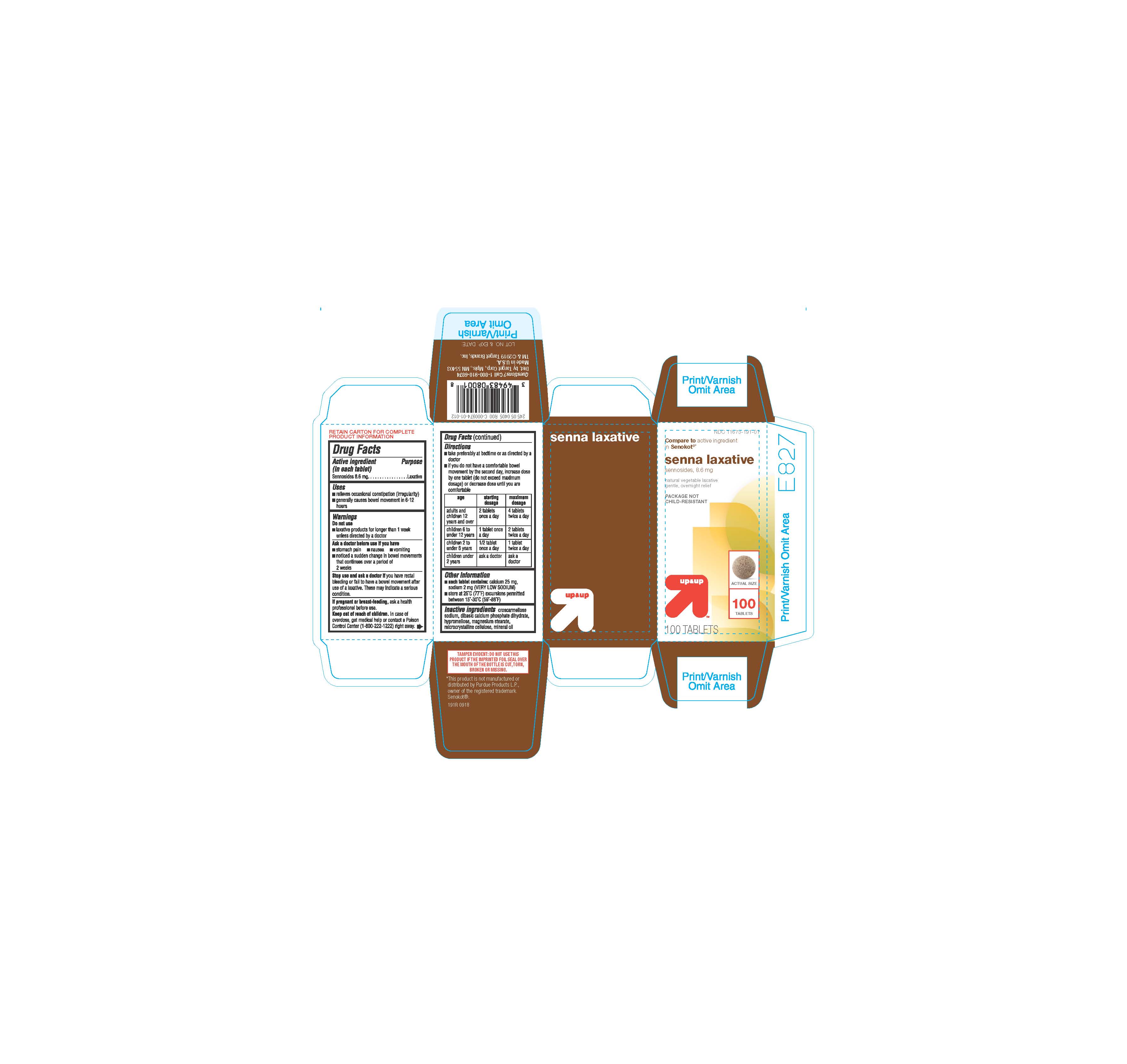
INACTIVE INGREDIENTS
croscarmellose sodium, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil
|
Directions: Take preferably at bedtime or as directed by a doctor; if you do not have a comfortable bowel movement by the second day, increase dose by one tablet (do not exceed maximum dosage) or decrease dose until you are comfortable. Adults and children 12 years and over - starting dosage: 2 tablets once a day Maximum dosage: 4 tablets twice a day Children 6 to under 12 years - starting dosage: 1 tablet once a day Maximum dosage: 2 tablets twice a day Children 2 to under 6 years - starting dosage: 1/2 tablet once a day Maximum dosage: 1 tablet twice a day Children under 2 years - ask a doctor |