FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% is a histamine H1 receptor antagonist indicated for the treatment of itching associated with signs and symptoms of allergic conjunctivitis.
2 DOSAGE AND ADMINISTRATION
Instill one drop of BEPREVE™ into the affected eye(s) twice a day (BID).
5 WARNINGS AND PRECAUTIONS
5.1 Contamination of Tip and Solution
To minimize contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
5.2 Contact Lens Use
Patients should be advised not to wear a contact lens if their eye is red. BEPREVE™ should not be used to treat contact lens-related irritation.
BEPREVE™ should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of BEPREVE™. The preservative in BEPREVE™, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of BEPREVE™.
6 ADVERSE REACTIONS
The most common reported adverse reaction occurring in approximately 25% of subjects was a mild taste following instillation. Other adverse reactions occurring in 2-5% of subjects were eye irritation, headache, and nasopharyngitis.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: Teratogenicity studies have been performed in animals. Bepotastine besilate was not found to be teratogenic in rats during organogenesis and fetal development at oral doses up to 200 mg/kg/day (representing a systemic concentration approximately 3,300 times that anticipated for topical ocular use in humans), but did show some potential for causing skeletal abnormalities at 1,000 mg/kg/day. There were no teratogenic effects seen in rabbits at oral doses up to 500 mg/kg/day given during organogenesis and fetal development (>13,000 times the dose in humans on a mg/kg basis). Evidence of infertility was seen in rats given oral bepotastine besilate 1,000 mg/kg/day however, no evidence of infertility was observed in rats given 200 mg/kg/day (approximately 3,300 times the topical ocular use in humans). The concentration of radiolabeled bepotastine besilate was similar in fetal liver and maternal blood plasma following a single 3 mg/kg oral dose. The concentration in other fetal tissues was one-third to one-tenth the concentration in maternal blood plasma.
An increase in stillborns and decreased growth and development were observed in pups born from rats given oral doses of 1,000 mg/kg/day during perinatal and lactation periods. There were no observed effects in rats treated with 100 mg/kg/day.
There are no adequate and well-controlled studies of bepotastine besilate in pregnant women. Because animal reproduction studies are not always predictive of human response, BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Following a single 3 mg/kg oral dose of radiolabeled bepotastine besilate to nursing rats 11 days after delivery, the maximum concentration of radioactivity in milk was 0.40 μg eq/mL 1 hour after administration; at 48 hours after administration the concentration was below detection limits. The milk concentration was higher than the maternal blood plasma concentration at each time of measurement.
It is not known if bepotastine besilate is excreted in human milk. Caution should be exercised when BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% is administered to a nursing woman.
8.4 Pediatric Use
Safety and efficacy of BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% have not been established in pediatric patients under 2 years of age. Efficacy in pediatric patients under 10 years of age was extrapolated from clinical trials conducted in pediatric patients greater than 10 years of age and from adults.
11 DESCRIPTION
BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% is a sterile, topically administered drug for ophthalmic use. Each mL of BEPREVE™ contains 15 mg bepotastine besilate.
Bepotastine besilate is designated chemically as (+) -4-[[(S)-p-chloro-alpha -2-pyridylbenzyl]oxy]-1-piperidine butyric acid monobenzenesulfonate. The chemical structure for bepotastine besilate is:
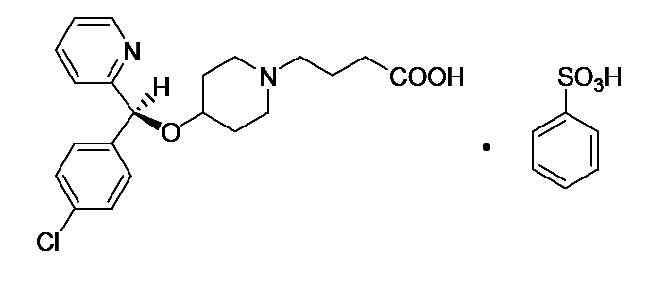
Bepotastine besilate is a white or pale yellowish crystalline powder. The molecular weight of bepotastine besilate is 547.06 daltons. BEPREVE™ ophthalmic solution is supplied as a sterile, aqueous 1.5% solution, with a pH of 6.8.
The osmolality of BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% is approximately 290 mOsm/kg.
Each mL of BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% contains:
Active: Bepotastine besilate 15 mg (equivalent to 10.7 mg bepotastine)
Preservative: benzalkonium chloride 0.005%
Inactives: monobasic sodium phosphate dihydrate, sodium chloride, sodium hydroxide to adjust pH, and water for injection, USP.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bepotastine is a topically active, direct H1-receptor antagonist and an inhibitor of the release of histamine from mast cells.
12.3 Pharmacokinetics
Absorption: The extent of systemic exposure to bepotastine following topical ophthalmic administration of bepotastine besilate 1% and 1.5% ophthalmic solutions was evaluated in 12 healthy adults. Following one drop of 1% or 1.5% bepotastine besilate ophthalmic solution to both eyes four time daily (QID) for seven days, bepotastine plasma concentrations peaked at approximately one to two hours post-instillation. Maximum plasma concentration for the 1% and 1.5% strengths were 5.1 ± 2.5 ng/mL and 7.3 ± 1.9 ng/mL, respectively. Plasma concentration at 24 hours post-instillation were below the quantifiable limit (2ng/mL) in 11/12 subjects in the two dose groups.
Dtistribution: The extent of protein binding of bepotastine is approximately 55% and independent of bepotastine concentration.
Metabolism:In vitro metabolism studies with human liver microsomes demonstrated that bepotastine is minimally metabolized by CYP450 isozymes.
In vitro studies demonstrated that bepotastine besilate does not inhibit the metabolism of various cytochrome P450 substrate via inhibition of CYP3A4, CYP2C9, and CYP2C19. The effect of bepotastine besilate on the metabolism of substrates of CYP1A2, CYP2C8, CYP2D6 was not studied. Bepotastine besilate has a low potential for drug interaction via inhibition of CYP3A4, CYP2C9, and CYP2C19.
Excretion: The main route of elimination of bepotastine besilate is urinary excretion (with approximately 75-90% excreted unchanged in urine).
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term dietary studies in mice and rats were conducted to evaluate the carcinogenic potential of bepotastine besilate. Bepotastine besilate did not significantly induce neoplasms in mice receiving a nominal dose of up to 200 mg/kg/day for 21 months or rats receiving a nominal dose of up to 97 mg/kg/day for 24 months. These dose levels represent systemic exposures approximating 350 and 200 times that achieved with human topical ocular use.
The no observable adverse effect levels for bepotastine besilate based on nominal dose levels in carcinogenicity tests were 18.7 to 19.9 mg/kg/day in mice and 9.6 to 9.8 mg/kg/day in rats (representing exposure margins of approximately 60 and 20 times the systemic exposure anticipated for topical use in humans).
There was no evidence of genotoxicity in the Ames test, in CHO cells (chromosome aberrations), in mouse hepatocytes (unscheduled DNA synthesis), or in the mouse micronucleus test.
When oral bepotastine was administered to male and female rats at doses up to 1,000 mg/kg/day, there was a slight reduction in fertility index and surviving fetuses. Infertility was not seen in rats given 200 mg/kg/day oral bepotastine besilate (approximately 3,300 times the systemic concentration anticipated for topical ocular use in humans).
14 CLINICAL STUDIES
Clinical efficacy was evaluated in 2 conjunctival allergen challenge (CAC) studies (237 patients). BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% was more effective than its vehicle for relieving ocular itching induced by an ocular allergen challenge, both at a CAC 15 minutes post-dosing and a CAC 8 hours post dosing of BEPREVE™.
The safety of BEPREVE™ was evaluated in a randomized clinical study of 861 subjects over a period of 6 weeks.
16 HOW SUPPLIED/STORAGE AND HANDLING
BEPREVE™ (bepotastine besilate ophthalmic solution) 1.5% is supplied in a white low density polyethylene plastic squeeze bottle with a white controlled dropper tip and a white polypropylene cap in the following size:
10 mL (NDC 54868-6299-0)
STORAGE
Store at 15º – 25ºC (59º – 77ºF).
17 PATIENT COUNSELING INFORMATION
17.2 Sterility of Dropper Tip
Patients should be advised to not touch dropper tip to any surface, as this may contaminate the contents.
17.3 Concomitant Use of Contact Lenses
Patients should be advised not to wear a contact lens if their eye is red. Patients should be advised that BEPREVE™ should not be used to treat contact lens-related irritation.
Patients should also be advised to remove contact lenses prior to instillation of BEPREVE™. The preservative in BEPREVE™, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of BEPREVE™.
Manufactured for: ISTA Pharmaceuticals®, Inc.
Irvine, CA
92618
By: Bausch & Lomb Incorporated
Tampa, FL
33637
Under license from:
Senju Pharmaceutical Co.,
Ltd.
Osaka, Japan 541-004
® and ™ marks owned by ISTA
Pharmaceuticals, Inc.
U.S. Patents: 6,307,052; 6,780,877
©2009
ISTA Pharmaceuticals, Inc.
Relabeling of additional barcode by:
Physicians Total Care, Inc.
Tulsa, OK 74146
