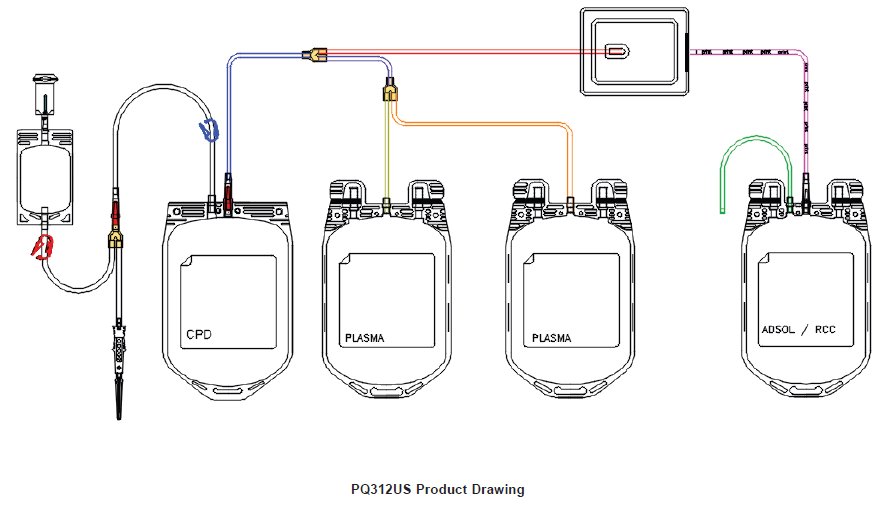
Contains Sample Diversion System for the collection of unanticoagulated whole blood samples for laboratory testing.
Caution: The Composelect System (PQ312US) is not FDA approved for manufacturing Platelets in the United States.
Store at Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
Definition of “Controlled Room Temperature”:
“A temperature maintained thermostatically that encompasses the usual and customary working environment of 20° to 25° C (68° to 77° F); that results in a mean kinetic temperature calculated to be not more than 25° C; and that allows for excursions between 15° C and 30° C (59° and 86° F) that are experienced in pharmacies, hospitals, and warehouses. Provided the mean kinetic temperature remains in the allowed range, transient spikes up to 40° C are permitted as long as they do not exceed 24 hours ... The mean kinetic temperature is a calculated value that may be used as an isothermal storage temperature that simulates the non isothermal effects of storage temperature variations.”
Reference: United States Pharmacopeia, General Notices. United States Pharmacopeial Convention, Inc. 12601 Twinbrook Parkway, Rockville, MD.
Description
The blood bag system has a sterile, non-pyrogenic fluid path and is intended for single use only.
Contains sample pouch for the collection of unanticoagulated whole blood samples for laboratory testing. Integral filter unit intended for leukocyte reduction of AS-1 red blood cells:
- •
- At ambient temperature up to 8 hours after blood collection.
- •
- At refrigerated temperature (1° to 6°C) up to 3 days after blood collection if AS-1 red blood cells are prepared within 3 days after whole blood collection.
Leukocyte reduced red blood cells may then be stored for up to 42 days after collection.
General Precautions
- •
- Do not squeeze or fold the filter
- •
- Cannulae must be opened by hand. Do not use any manual hand opening tools, such as the Fenwal Cannula Cracker.
- •
- Inadequate clamping of the donation line can cause non-sterile air to enter the blood bag once the needle cover has been removed
- •
- Do not use the Composelect System (PQ312US) for the manufacture of Platelets. FDA has not approved this container for manufacturing Platelets in the United States.
Directions for Use
Pre-collection Inspection
Examine the collection container and storage containers. Do not use the container if you observe any of the following:
- •
- Visible damage, defects or non-conformity to the product representing a risk to the integrity of the system
- •
- Appearance and translucence of the solutions
- •
- Kinks and large dents in the tubing
- •
- Damaged or loose components of the product
- •
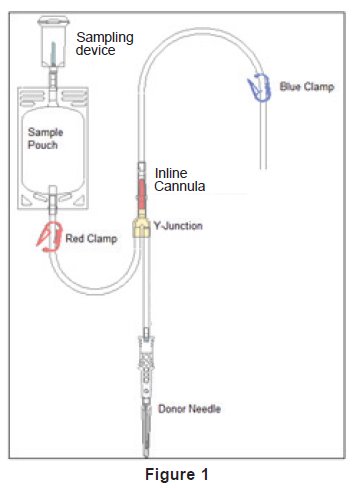
- In-line cannula is broken and/or anticoagulant is present in the sample pouch or in the tubing from the in-line cannula to the sample pouch and donor needle (see Figure 1).
Note: Condensation in the empty tubing of the Composelect System is expected as a result of the sterilization process.
Collection Procedure
Use aseptic technique.
1. Identify Composelect System using appropriate donor identification system.
Note: The satellite containers are not intended for storage of Platelets. Do not label for Platelet storage
2. Adjust donor scale to desired collection weight and position the blood bag system on the donor scale as far as possible below donor arm.
Note: When using a Composelect System on a donor scale, ensure that the filter is not placed between the bags as this could cause improper mixing of the blood with anticoagulant.
3. Unwind the tubing and ensure it is torsion-free to prevent the needle from turning while drawing blood. See Figure 1.
4. Close only the blue clamp on the donor tubing. See Figure 1.
Notes:
- •
- Do not close the red clamp to the sample pouch; it is a non-reopenable clamp. See Figure 1.
- •
- Ensure that the sample pouch remains below the donor’s arm.
5. Following facility procedures, disinfect site of venipuncture, then apply pressure to donor’s arm.
6. Remove Fenwal HighFlo1 needle cover per instructions below:
- a) Holding the hub and cover near tamper-evident seal, twist cover and hub in opposite directions to break seal.
- b) Remove and discard needle cover, being careful not to drag the cover across the needle point.
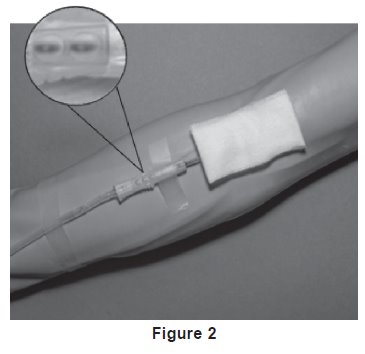
7. Following facility procedures, perform venipuncture and appropriately secure donor needle and/or tubing. When good blood flow is established, stabilize the front of the needle guard to arm with tape (see Figure 2).
8. As the sample pouch is filling, break the in-line cannula below the Y-junction. See Figure 1.
Note: To completely break the in-line cannula, grasp with both hands. Snap it at a 90° angle in one direction and then bend it at a 180° angle in the opposite direction.
Precaution: Failure to break the in-line cannula completely may result in restricted blood flow.
9. Allow the sample pouch to fill with blood according to facility procedures. Monitor blood flow into the sample pouch.
Precautions:
- •
- Do not elevate or squeeze the sample pouch as this could cause blood to backflow from the sample pouch into the collection system.
- •
- Once the sample pouch is filled to desired volume, complete steps 10 – 16 within approximately 4 minutes to avoid possible clot formation in the tubing and/or sample pouch.
10. After filling the sample pouch, close the red clamp on the line towards the sample pouch.
11. Open the blue clamp on donor tubing to begin collection. See Figure 1.
Note: Mix blood and anticoagulant in the primary container immediately, at several intervals during collection, and immediately after collection.
12. Once collection has begun, hermetically seal tubing between sample pouch and the red clamp.
13. To collect samples, open the cap of the sample access device, then hold the sample pouch in an upright position with the access device pointing downwards.
14. Directly align the vacuum sample tube with the internal needle in the access device. Insert vacuum sample tube into device until the stopper is punctured.
15. Allow vacuum sample tube to fill with blood then remove from the access device.
Note: Don’t squeeze the sample pouch.
16. Repeat steps 14 and 15 until the desired number of vacuum sample tubes have been filled.
17. Collect 500 mL ±10% volume of blood.
Note: The volume of anticoagulant is sufficient for the blood collection indicated on the Composelect System ± 10%.
18. After the collection has been completed, close the blue clamp on the donor tubing.
Precaution: Once the desired blood volume is collected, complete steps 19 to 23 within approximately 4 minutes to avoid possible clot formation in the tubing.
19. Release pressure on donor’s arm.
20. Hermetically seal donor tubing between the in-line cannula and the primary container.
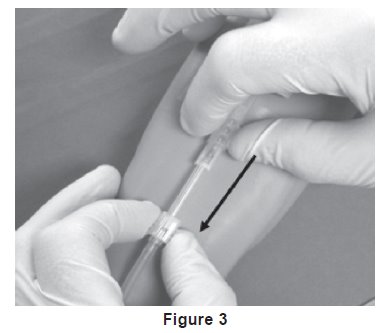
21. Withdrawal of Needle (see Figure 3).
Precaution: The needle guard must be held stationary while the needle is withdrawn into it.
a) Place folded sterile gauze over puncture site and hold in place with finger tip without exerting pressure.
b) Hold sides of needle guard near the front, between the index finger and thumb. Pull the hub back smoothly until the needle is completely enclosed and securely locked into the needle guard.
c) Confirm the needle is completely enclosed and securely locked into the needle guard.
22. Grip the retracted needle by the needle hub and place the needle into the sample access device, then close the cap to secure the needle.
23. Remove and discard the sampling system and needle guard into an appropriate biohazardous waste container following facility procedures. If donor tubing is also to be discarded, hermetically seal donor tubing directly above the primary container and remove.
24. If the donor tubing is not hermetically sealed directly above the primary container, then strip the blood from the remaining donor tubing into the primary container and mix. If desired, allow tubing to refill.
25. Follow your facility procedure to place the unit into appropriate temporary storage at ambient temperature or with sufficient coolant to lower the temperature continuously toward 1-10 °C until delivered to the component processing facility.
Component Preparation
Centrifugation and Plasma Separation
Notes:
- •
- Fresh frozen plasma should be separated from the red blood cells and placed in the freezer at -18°C or colder within 8 hours after blood collection.
- •
- Adsol red cell preservation solution should be added to the red blood cells immediately after the removal of plasma.
- •
- Preparation of AS-1 red blood cells may vary depending on processing option selected:
- a) Within 8 hours of blood collection if whole blood is held at ambient temperature.
- b) Within 3 days of blood collection if whole blood is stored at 1 °C - 6 °C. At the appropriate time, prepare the Composelect System for centrifugation.
26. Thoroughly mix the primary container end over end.
27. Load the Composelect System into a centrifuge cup per the instructions on page 4.
Notes:
- •
- This guide is one method for centrifuge cup loading. The specific stacking order and methods may vary depending on the workstation setup, centrifuge equipment, and facility-specific Standard Operating Procedures.
- •
- It is important to pack the filter properly in the centrifugation cup to avoid damage to the filter during centrifugation.
28. After loading the Composelect System into the centrifuge cup, perform centrifugation according to facility procedures.
29. Following centrifugation, remove containers from the centrifuge cup, taking care not to disturb the red blood cell / plasma interface.
30. Place primary container in a plasma extractor and apply pressure. Clamp off tubing above the filter to prevent plasma flow into filter during plasma transfer. Clamp off tubing below Y-junction leading to secondary Transfer Pack container not to be filled with plasma. Open the cannula on the top of the primary container to transfer plasma into the empty transfer pack container.
31. When the desired amount of plasma has been removed, clamp the tubing between the plasma container and the Y-junction closest to the plasma container and release pressure on the primary container.
32. Hermetically seal and remove satellite containers.
Addition of Adsol to Red Cells
33. Suspend the Adsol red cell preservation solution container, above the primary container ensuring that the primary container remains below the level of the filter during prime.
34. Open the cannula on the Adsol solution container and remove the clamp between the primary container and the filter.
35. Allow the Adsol to drain through the filter into the primary container.
36. After prime is complete, clamp the tubing between the filter and primary container.
37. Mix the Adsol red cell preservation solution and red cells thoroughly.
Notes:
- •
- If the Adsol red cell preservation solution is not added to the red cells, ensure appropriate labeling of the red cell container. Also ensure that tubing with unique segment numbers are attached, as the primary container does not include tubing with segment numbers. Manufacture of CPD red cells for transfusion must include proper labeling of the container, as well as attached tubing, with unique segment numbers.
- •
- A CPD red cell without Adsol red cell preservation solution may be stored between 1º and 6º C up to 21 days after collection. The attached Bioflex RC filter should not be used to leukoreduce a CPD red cell product without Adsol red cell preservation solution and should be detached from the primary container.
- •
- CPD Red Blood Cells without Adsol can be leukoreduced using an appropriate “dock-on” filter.
Integral Red Cell Filtration
Precaution: Red blood cell products collected from certain donors may have extended filtration times and the potential for ineffective leukoreduction.
38. Mix unfiltered AS-1 red blood cells thoroughly. Invert the unfiltered AS-1 red blood cells and hang the filter set such that the filter remains vertical. To achieve maximum flow rate, allow the set to hang to full length. The storage container must remain below the level of the filter during filtration.
39. Filtration must be initiated within 8 hours after collection if Red Blood cells are maintained at ambient temperature and filtration is performed at ambient temperature or within 3 days after collection if Red Blood Cells are stored at 1°C - 6°C and filtration is performed at 1°C - 6°C
40. Inspect all tubing to ensure it hangs freely without kinks.
41. Remove the clamp above the filter to start filtration.
Note: Manual or mechanical pressure should not be used to increase the flow rate through the filter. Tubing below the filter should not be stripped at any time during the filtration process.
Note: If filtration of red cells is initiated at ambient temperature, the filtration process can be completed at either ambient or refrigerated temperature prior to storing the red cells between 1° and 6° C. However, for optimal filtration time, it is recommended to complete the filtration at ambient temperature.
42. When filtration is complete, air can be observed in the inlet side of the filter. Hermetically seal and detach the tubing below the filter.
43. Make segments on the post-filter tubing and leave segments attached to the leukoreduced red cell storage container. QC samples may be prepared by stripping the red blood cells in the tubing, thoroughly mixing the red blood cells, then refilling the tubing and sealing the segments. Discard the filter and the empty primary container.
44. Store the AS-1 red blood cells, leukocytes reduced between 1º and 6° C.
45. Transfuse the red cells within 42 days of collection.
46. Quality control should be per FDA “Guidance for Industry - Pre- Storage Leukocyte Reduction of Whole blood and Blood Components Intended for Transfusion”, current issue.
Warning: Failure to achieve closed system processing conditions negates the extended storage claim and the red blood cell product must be transfused within 24 hours. If the integrity of the Red Blood Cell product is compromised, the product must be labeled appropriately as Open System.
Dispose of waste in appropriate biohazard container or according to local regulatory requirements.
1 Van der Meer, P.F., & de Korte, D. “Increase of blood donation speed by optimizing the needle-to-tubing connection: an application of donation software.” Vox Sanguinis 2009, 97: 21-25
Fresenius Kabi AG
61346 Bad Homburg
Germany
www.fresenius-kabi.com
1-800-933-6925
© 2022 Fresenius Kabi AG. All rights reserved.
All trademarks referred to are property of their respective owners.
33827 11/2022