CII
Rx only
WARNING: HYDROMORPHONE HYDROCHLORIDE TABLETS USP, 2 mg, 4 mg, and 8 mg CONTAIN HYDROMORPHONE, WHICH IS A POTENT SCHEDULE II CONTROLLED OPIOID AGONIST. SCHEDULE II OPIOID AGONISTS, INCLUDING MORPHINE, OXYMORPHONE, OXYCODONE, FENTANYL, AND METHADONE, HAVE THE HIGHEST POTENTIAL FOR ABUSE AND RISK OF PRODUCING RESPIRATORY DEPRESSION. ALCOHOL, OTHER OPIOIDS AND CENTRAL NERVOUS SYSTEM DEPRESSANTS (SEDATIVE-HYPNOTICS) POTENTIATE THE RESPIRATORY DEPRESSANT EFFECTS OF HYDROMORPHONE, INCREASING THE RISK OF RESPIRATORY DEPRESSION THAT MIGHT RESULT IN DEATH.
DESCRIPTION
Hydromorphone hydrochloride, a hydrogenated ketone of morphine, is an opioid analgesic.
The chemical name of hydromorphone hydrochloride is 4,5α-epoxy-3-hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula of hydromorphone hydrochloride is:
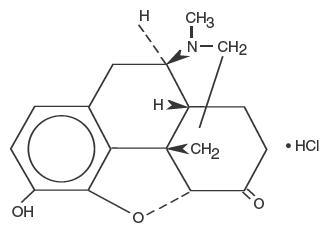
C17H19NO3 • HCl M.W. 321.8
Each hydromorphone hydrochloride tablet USP, 2 mg contains 2 mg hydromorphone hydrochloride. Each hydromorphone hydrochloride tablet USP, 4 mg contains 4 mg hydromorphone hydrochloride. Each hydromorphone hydrochloride tablet USP, 8 mg contains 8 mg hydromorphone hydrochloride. In addition, the tablets include anhydrous lactose, lactose monohydrate, and magnesium stearate.
CLINICAL PHARMACOLOGY
Hydromorphone hydrochloride is a pure opioid agonist with the principal therapeutic activity of analgesia. A significant feature of the analgesia is that it can occur without loss of consciousness.
Opioid analgesics also suppress the cough reflex and may cause respiratory depression, mood changes, mental clouding, euphoria, dysphoria, nausea, vomiting and electroencephalographic changes. Many of the effects described below are common to this class of mu-opioid agonist analgesics which includes morphine, oxycodone, hydrocodone, codeine and fentanyl. In some instances, data may not exist to distinguish the effects of hydromorphone hydrochloride tablets from those observed with other opioid analgesics. However, in the absence of data to the contrary, it is assumed that hydromorphone hydrochloride tablets would possess all the actions of mu-agonist opioids.
Central Nervous System
The precise mode of analgesic action of opioid analgesics is unknown. However, specific CNS opiate receptors have been identified. Opioids are believed to express their pharmacological effects by combining with these receptors.
Hydromorphone depresses the cough reflex by direct effect on the cough center in the medulla.
Hydromorphone depresses the respiratory reflex by a direct effect on brain stem respiratory centers.
The mechanism of respiratory depression also involves a reduction in the responsiveness of the brain stem respiratory centers to increases in carbon dioxide tension.
Hydromorphone causes miosis. Pinpoint pupils are a common sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in the setting of hydromorphone hydrochloride overdose.
Gastrointestinal Tract and Other Smooth Muscle
Gastric, biliary and pancreatic secretions are decreased by opioids such as hydromorphone. Hydromorphone causes a reduction in motility associated with an increase in tone in the gastric antrum and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, and tone may be increased to the point of spasm. The end result is constipation. Hydromorphone can cause a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi.
PHARMACOKINETICS AND METABOLISM
The analgesic activity of hydromorphone hydrochloride is due to the parent drug, hydromorphone. Hydromorphone is rapidly absorbed from the gastrointestinal tract after oral administration and undergoes extensive first-pass metabolism. Exposure of hydromorphone (Cmax and AUC0-24) is dose-proportional at a dose range of 2 and 8 mg. In vivo bioavailability following single-dose administration of the 8 mg tablet is approximately 24% (coefficient of variation 21%).
Absorption
After oral administration of hydromorphone hydrochloride 8 mg tablets, peak plasma hydromorphone concentrations are generally attained within 1/2 to 1-hour.
| Mean (%cv) | ||||
|---|---|---|---|---|
| Dosage Form | Cmax
(ng) | Tmax
(hrs) | AUC (ng*hr/mL) | T1/2
(hrs) |
|
8 mg Tablet | 5.5 (33%) | 0.74 (34%) | 23.7 (28%) | 2.6 (18%) |
Food effects
In a study conducted with a single 8 mg dose of hydromorphone (2 mg hydromorphone hydrochloride IR tablets), food lowered Cmax by 25%, prolonged Tmax by 0.8 hour, and increased AUC by 35%. The effects may not be clinically relevant.
Distribution
At therapeutic plasma levels, hydromorphone is approximately 8 to 19% bound to plasma proteins. After an intravenous bolus dose, the steady state of volume distribution [mean (%cv)] is 302.9 (32%) liters.
Metabolism
Hydromorphone is extensively metabolized via glucuronidation in the liver, with greater than 95% of the dose metabolized to hydromorphone-3-glucuronide along with minor amounts of 6-hydroxy reduction metabolites.
Elimination
Only a small amount of the hydromorphone dose is excreted unchanged in the urine. Most of the dose is excreted as hydromorphone-3-glucuronide along with minor amounts of 6-hydroxy reduction metabolites. The systemic clearance is approximately 1.96 (20%) liters/minute. The terminal elimination half-life of hydromorphone after an intravenous dose is about 2.3 hours.
Special Populations
Hepatic Impairment
After oral administration of hydromorphone at a single 4 mg dose (2 mg hydromorphone hydrochloride IR tablets), mean exposure to hydromorphone (Cmax and AUC∞) is increased 4-fold in patients with moderate (Child-Pugh Group B) hepatic impairment compared with subjects with normal hepatic function. Due to increased exposure of hydromorphone, patients with moderate hepatic impairment should be started at a lower dose and closely monitored during dose titration.
Pharmacokinetics of hydromorphone in severe hepatic impairment patients has not been studied.
Further increase in Cmax and AUC of hydromorphone in this group is expected. As such, starting dose should be even more conservative.
Renal Impairment
After oral administration of hydromorphone at a single 4 mg dose (2 mg hydromorphone hydrochloride IR Tablets), exposure to hydromorphone ( Cmax and AUC0-48) is increased in patients with impaired renal function by 2-fold in moderate (CLcr = 40 - 60 mL/min) and 3-fold in severe (CLcr < 30 mL/min) renal impairment compared with normal subjects (CLcr > 80 mL/min). In addition, in patients with severe renal impairment hydromorphone appeared to be more slowly eliminated with longer terminal elimination half-life (40 hr) compared to patients with normal renal function (15 hr).
Patients with moderate renal impairment should be started on a lower dose. Starting doses for patients with severe renal impairment should be even lower. Patients with renal impairment should be closely monitored during dose titration.
CLINICAL TRIALS
Analgesic effects of single doses of hydromorphone hydrochloride oral liquid administered to patients with post-surgical pain have been studied in double-blind controlled trials. In one study, both 5 mg and 10 mg of hydromorphone hydrochloride oral liquid provided significantly more analgesia than placebo. In another trial, 5 mg and 10 mg of hydromorphone hydrochloride oral liquid were compared to 30 mg and 60 mg of morphine sulfate oral liquid. The pain relief provided by 5 mg and 10 mg hydromorphone hydrochloride oral liquid was comparable to 30 mg and 60 mg oral morphine sulfate, respectively.
INDICATIONS AND USAGE
Hydromorphone hydrochloride tablets are indicated for the management of pain in patients where an opioid analgesic is appropriate.
CONTRAINDICATIONS
Hydromorphone hydrochloride tablets are contraindicated in: patients with known hypersensitivity to hydromorphone, patients with respiratory depression in the absence of resuscitative equipment, and in patients with status asthmaticus. Hydromorphone hydrochloride tablets are also contraindicated for use in obstetrical analgesia.
WARNINGS
Respiratory Depression
Respiratory depression is the chief hazard of hydromorphone hydrochloride tablets. Respiratory depression is more likely to occur in the elderly, in the debilitated, and in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation.
Hydromorphone hydrochloride tablets should be used with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale, patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or in patients with preexisting respiratory depression. In such patients even usual therapeutic doses of opioid analgesics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
Hydromorphone Hydrochloride Tablets, USP contain hydromorphone, which is a potent Schedule II controlled opioid agonist. Schedule II opioid agonists, including morphine, oxymorphone, oxycodone, fentanyl, and methadone, have the highest potential for abuse and risk of producing respiratory depression. Alcohol, other opioids and central nervous system depressants (sedative-hypnotics) potentiate the respiratory depressant effects of hydromorphone, increasing the risk of respiratory depression that might result in death.
Misuse, Abuse, and Diversion of Opioids
Hydromorphone is an opioid agonist of the morphine-type. Such drugs are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
Hydromorphone hydrochloride can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing hydromphone hydrochloride tablets in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion. Prescribers should monitor all patients receiving opioids for signs of abuse, misuse, and addiction. Furthermore, patients should be assessed for their potential for opioid abuse prior to being prescribed opioid therapy. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse) or mental illness (e.g., depression). Opioids may still be appropriate for use in these patients, however, they will require intensive monitoring for signs of abuse.
Hydromorphone hydrochloride tablets have been reported as being abused by crushing, chewing, snorting, or injecting the dissolved product. These practices pose a significant risk to the abuser that could result in overdose or death (see WARNINGS and DRUG ABUSE AND DEPENDENCE).
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain.
Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Alcohol and Drugs of Abuse
Hydromorphone may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression.
Neonatal Withdrawal Syndrome
Infants born to mothers physically dependent on hydromorphone hydrochloride will also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms. (see DRUG ABUSE AND DEPENDENCE).
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of hydromorphone hydrochloride tablets with carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or preexisting increase in intracranial pressure. Opioid analgesics including hydromorphone hydrochloride tablets may produce effects on pupillary response and consciousness which can obscure the clinical course and neurologic signs of further increase in intracranial pressure in patients with head injuries.
Hypotensive Effect
Opioid analgesics, including hydromorphone hydrochloride tablets, may cause severe hypotension in an individual whose ability to maintain blood pressure has already been compromised by a depleted blood volume, or a concurrent administration of drugs such as phenothiazines or general anesthetics (see PRECAUTIONS Drug Interactions). Therefore, hydromorphone hydrochloride tablets should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
PRECAUTIONS
Special Risk Patients
Hydromorphone hydrochloride tablets should be given with caution and the initial dose should be reduced in the elderly or debilitated and those with severe impairment of hepatic, pulmonary or renal functions; myxedema or hypothyroidism; adrenocortical insufficiency (e.g., Addison's Disease); CNS depression or coma; toxic psychoses; prostatic hypertrophy or urethral stricture; gall bladder disease; acute alcoholism; delirium tremens; kyphoscoliosis or following gastrointestinal surgery.
The administration of opioid analgesics including hydromorphone hydrochloride tablets may obscure the diagnoses or clinical course in patients with acute abdominal conditions and may aggravate preexisting convulsions in patients with convulsive disorders.
Reports of mild to severe seizures and myoclonus have been reported in severely compromised patients, administered high doses of parenteral hydromorphone, for cancer and severe pain. Opioid administration at very high doses is associated with seizures and myoclonus in a variety of diseases where pain control is the primary focus.
Use in Drug and Alcohol Dependent Patients
Hydromorphone hydrochloride tablets should be used with caution in patients with alcoholism and other drug dependencies due to the increased frequency of opioid tolerance, dependence, and the risk of addiction observed in these patient populations. Abuse of hydromorphone hydrochloride tablets in combination with other CNS depressant drugs can result in serious risk to the patient.
Hydromorphone is an opioid with no approved use in the management of addictive disorders.
Use in Ambulatory Patients
Hydromorphone hydrochloride tablets may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g. driving, operating machinery). Patients should be cautioned accordingly. Hydromorphone hydrochloride tablets may produce orthostatic hypotension in ambulatory patients.
Tolerance and Physical Dependence
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Physical dependence and tolerance are not unusual during chronic opioid therapy.
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, mydriasis. Other symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
In general, opioids used regularly should not be abruptly discontinued.
Information for Patients/Caregivers
Patients receiving hydromorphone hydrochloride tablets or their caregivers should be given the following information by the physician, nurse, or pharmacist:
- Patients should be aware that hydromorphone hydrochloride tablets contain hydromorphone, which is a morphine-like substance and which could cause severe adverse effects including respiratory depression and even death if not taken according to the prescriber's directions.
- Patients should be advised to report pain and adverse experiences occurring during therapy. Individualization of dosage is essential to make optimal use of this medication.
- Patients should be advised not to adjust the dose of hydromorphone hydrochloride tablets without consulting the prescribing professional.
- Patients should be advised that hydromorphone hydrochloride tablets may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating heavy machinery).
- Patients should not combine hydromorphone hydrochloride tablets with alcohol or other central nervous system depressants (sleep aids, tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur, resulting in serious injury or death.
- Women of childbearing potential who become, or are planning to become pregnant should be advised to consult their physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
- Patients should be advised that hydromorphone hydrochloride tablets are a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
- Patients should be advised that if they have been receiving treatment with hydromorphone hydrochloride tablets for more than a few weeks and cessation of therapy is indicated, it may be appropriate to taper the hydromorphone hydrochloride tablets dose, rather than abruptly discontinue it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a gradual discontinuation of the medication.
- Patients should be instructed to keep hydromorphone hydrochloride tablets in a secure place out of the reach of children. When hydromorphone hydrochloride tablets are no longer needed, the unused tablets should be destroyed by flushing down the toilet.
Drug Interactions
Drug Interactions with Other CNS Depressants
The concomitant use of other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers and alcohol may produce additive depressant effects. Respiratory depression, hypotension and profound sedation or coma may occur. When such combined therapy is contemplated, the dose of one or both agents should be reduced. Hydromorphone hydrochloride tablets should not be taken with alcohol. Opioid analgesics, including hydromorphone hydrochloride tablets, may enhance the action of neuromuscular blocking agents and produce an excessive degree of respiratory depression.
Interactions with Mixed Agonist/Antagonist Opioid Analgesics
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol, and buprenorphine) should be administered with caution to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic such as hydromorphone. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of hydromorphone and/or may precipitate withdrawal symptoms in these patients.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted in animals.
Hydromorphone was not mutagenic in the in vitro Ames reverse mutation assay or the human lymphocyte chromosome aberration assay. Hydromorphone was not clastogenic in the in vivo mouse micronucleus assay.
No effects on fertility, reproductive performance, or reproductive organ morphology were observed in male or female rats given oral doses up to 7 mg/kg/day, which is 3-fold higher than the human dose of 2-4 mg every 4 to 6 hours for the tablet on a body surface area basis.
PREGNANCY
PREGNANCY CATEGORY C
No effects on teratogenicity or embryotoxicity were observed in female rats given oral doses up to 7 mg/kg/day, which is approximately 3-fold higher than the human dose of 2-4 mg every 4 to 6 hours for the tablet on a body surface area basis. Hydromorphone produced skull malformations (exencephaly and cranioschisis) in Syrian hamsters given oral doses up to 20 mg/kg during the peak of organogenesis (gestation days 8-9). The skull malformations were observed at doses approximately 7-fold higher than the human dose of 2-4 mg every 4 to 6 hours for the tablet on a body surface area basis. There are no adequate and well-controlled studies of hydromorphone hydrochloride tablets in pregnant women.
Hydromorphone crosses the placenta, resulting in fetal exposure. Hydromorphone hydrochloride tablets should be used in pregnant women only if the potential benefit justifies the potential risk to the fetus (see Labor and Delivery and DRUG ABUSE AND DEPENDENCE).
Nonteratogenic effects
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal. Approaches to the treatment of this syndrome have included supportive care and, when indicated, drugs such as paregoric or phenobarbital.
Labor and Delivery
Hydromorphone hydrochloride tablets are contraindicated in Labor and Delivery (see CONTRAINDICATIONS).
Nursing Mothers
Low levels of opioid analgesics have been detected in human milk. As a general rule, nursing should not be undertaken while a patient is receiving hydromorphone hydrochloride tablets since it, and other drugs in this class, may be excreted in the milk.
Geriatric Use
Clinical studies of hydromorphone hydrochloride tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see INDIVIDUALIZATION OF DOSAGES and PRECAUTIONS).
ADVERSE REACTIONS
The major hazards of hydromorphone hydrochloride tablets include respiratory depression and apnea. To a lesser degree, circulatory depression, respiratory arrest, shock and cardiac arrest have occurred.
The most frequently observed adverse effects are light-headedness, dizziness, sedation, nausea, vomiting, sweating, flushing, dysphoria, euphoria, dry mouth, and pruritus. These effects seem to be more prominent in ambulatory patients and in those not experiencing severe pain.
Less Frequently Observed Adverse Reactions
General and CNS: Weakness, headache, agitation, tremor, uncoordinated muscle movements, alterations of mood (nervousness, apprehension, depression, floating feelings, dreams), muscle rigidity, paresthesia, muscle tremor, blurred vision, nystagmus, diplopia and miosis, transient hallucinations and disorientation, visual disturbances, insomnia, increased intracranial pressure
Cardiovascular: Flushing of the face, chills, tachycardia, bradycardia, palpitation, faintness, syncope, hypotension, hypertension.
Respiratory: Bronchospasm and laryngospasm
Gastrointestinal: Constipation, biliary tract spasm, ileus, anorexia, diarrhea, cramps, taste alteration
Genitourinary: Urinary retention or hesitancy, antidiuretic effects
Dermatologic: Urticaria, other skin rashes, diaphoresis.
OVERDOSAGE
Serious overdosage with hydromorphone hydrochloride tablets is characterized by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes bradycardia and hypotension. In serious overdosage, particularly following intravenous injection, apnea, circulatory collapse, cardiac arrest and death may occur.
In the treatment of overdosage, primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. A potentially serious oral ingestion, if recent, should be managed with gut decontamination. In unconscious patients with a secure airway, instill activated charcoal (30-100 g in adults, 1-2 g/kg in infants) via a nasogastric tube. A saline cathartic or sorbitol may be added to the first dose of activated charcoal.
Supportive measures (including oxygen, vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
The opioid antagonist, naloxone, is a specific antidote against respiratory depression which may result from overdosage, or unusual sensitivity to hydromorphone hydrochloride tablets. Therefore, an appropriate dose of this antagonist should be administered, preferably by the intravenous route, simultaneously with efforts at respiratory resuscitation. Naloxone should not be administered in the absence of clinically significant respiratory or circulatory depression. Naloxone should be administered cautiously to persons who are known, or suspected to be physically dependent on hydromorphone hydrochloride tablets. In such cases, an abrupt or complete reversal of narcotic effects may precipitate an acute withdrawal syndrome. Since the duration of action of hydromorphone hydrochloride tablets may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. Apply other supportive measures when indicated.
DOSAGE AND ADMINISTRATION
The usual starting dose for hydromorphone hydrochloride tablets is 2 mg to 4 mg, orally, every 4 to 6 hours. Appropriate use of the hydromorphone hydrochloride tablets must be decided by careful evaluation of each clinical situation.
A gradual increase in dose may be required if analgesia is inadequate, as tolerance develops, or if pain severity increases. The first sign of tolerance is usually a reduced duration of effect.
Patients with hepatic and renal impairment should be started on a lower starting dose (See CLINICAL PHARMACOLOGY: PHARMACOKINETICS and METABOLISM).
INDIVIDUALIZATION OF DOSAGE
The dosage of opioid analgesics like hydromorphone hydrochloride should be individualized for any given patient, since adverse events can occur at doses that may not provide complete freedom from pain.
Safe and effective administration of opioid analgesics to patients with acute or chronic pain depends upon a comprehensive assessment of the patient. The nature of the pain (severity, frequency, etiology, and pathophysiology) as well as the concurrent medical status of the patient will affect selection of the starting dosage.
In non-opioid-tolerant patients, therapy with hydromorphone is typically initiated at an oral dose of 2-4 mg every four hours, but elderly patients may require lower doses (see PRECAUTIONS - Geriatric Use).
In patients receiving opioids, both the dose and duration of analgesia will vary substantially depending on the patient's opioid tolerance. The dose should be selected and adjusted so that at least 3 to 4 hours of pain relief may be achieved. In patients taking opioid analgesics, the starting dose of hydromorphone hydrochloride tablets should be based on prior opioid usage. This should be done by converting the total daily usage of the previous opioid to an equivalent total daily dosage of oral hydromorphone hydrochloride tablets using an equianalgesic table (see below). For opioids not in the table, first estimate the equivalent total daily usage of oral morphine, then use the table to find the equivalent total daily dosage of hydromorphone hydrochloride tablets.
Once the total daily dosage of hydromorphone hydrochloride tablets has been estimated, it should be divided into the desired number of doses. Since there is individual variation in response to different opioid drugs, only 1/2 to 2/3 of the estimated dose of hydromorphone hydrochloride tablets calculated from equivalence tables should be given for the first few doses, then increased as needed according to the patient's response.
Since the pharmacokinetics of hydromorphone are affected in hepatic and renal impairment with a consequent increase in exposure, patients with hepatic and renal impairment should be started on a lower starting dose (See CLINICAL PHARMACOLOGY: PHARMACOKINETICS and METABOLISM).
In chronic pain, doses should be administered around-the-clock. A supplemental dose of 5-15% of the total daily usage may be administered every two hours on an "as-needed" basis.
Periodic reassessment after the initial dosing is always required. If pain management is not satisfactory and in the absence of significant opioid-induced adverse events, the hydromorphone dose may be increased gradually. If excessive opioid side effects are observed early in the dosing interval, the hydromorphone dose should be reduced. If this results in breakthrough pain at the end of the dosing interval, the dosing interval may need to be shortened. Dose titration should be guided more by the need for analgesia than the absolute dose of opioid employed.
| Nonproprietary Name | IM or SC Dose | ORAL Dose |
|---|---|---|
|
||
| Morphine Sulfate | 10 mg | 40-60 mg |
| Hydromorphone HCl | 1.3-2 mg | 6.5-7.5 mg |
| Oxymorphone HCl | 1-1.1 mg | 6.6 mg |
| Levorphanol tartrate | 2-2.3 mg | 4 mg |
| Meperidine, pethidine HCl | 75-100 mg | 300-400 mg |
| Methadone HCl | 10 mg | 10-20 mg |
DRUG ABUSE AND DEPENDENCE
Hydromorphone Hydrochloride Tablets, USP contain hydromorphone, a Schedule II controlled opioid agonist. Schedule II opioid substances which include morphine, oxycodone, oxymorphone, fentanyl, and methadone have the highest potential for abuse and risk of fatal overdose. Hydromorphone can be abused and is subject to criminal diversion.
Opioid analgesics may cause psychological and physical dependence. Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug. Physical dependence usually does not occur to a clinically significant degree until after several weeks of continued opioid usage, but it may occur after as little as a week of opioid use. Physical dependence and tolerance are separate and distinct from abuse and addiction.
Addiction is a chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
"Drug seeking" behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated "loss" of prescriptions, tampering with, forging or counterfeiting prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). "Doctor shopping" to obtain additional prescriptions is common among drug abusers, people suffering from untreated addiction and criminals seeking drugs to sell.
Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Since hydromorphone hydrochloride tablets may be diverted for non-medical use, careful record keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Hydromorphone hydrochloride tablets are intended for oral use only. Misuse or abuse of hydromorphone hydrochloride tablets pose a risk of overdose and death. This risk is increased with concurrent abuse of alcohol and other CNS depressants. Parenteral drug abuse can potentially result in local tissue necrosis, infection, pulmonary granulomas, and increased risk of endocarditis and valvular heart injury. In addition, parenteral abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
SAFETY AND HANDLING INSTRUCTIONS
Hydromorphone hydrochloride tablets pose little risk of direct exposure to health care personnel and should be handled and disposed of prudently in accordance with hospital or institutional policy. Significant absorption from dermal exposure is unlikely; accidental dermal exposure to hydromorphone hydrochloride should be treated by removal of any contaminated clothing and rinsing the affected area with cool water. Patients and their families should be instructed to flush any hydromorphone hydrochloride tablets that are no longer needed.
Access to abusable drugs such as hydromorphone hydrochloride tablets presents an occupational hazard for addiction in the health care industry. Routine procedures for handling controlled substances developed to protect the public may not be adequate to protect health care workers. Implementation of more effective accounting procedures and measures to restrict access to drugs of this class (appropriate to the practice setting) may minimize the risk of self-administration by health care providers.
HOW SUPPLIED
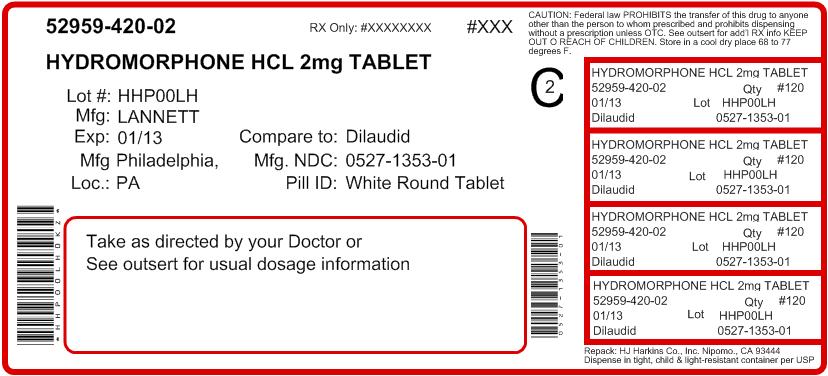
Hydromorphone Hydrochloride Tablets USP, 2 mg are white, round, flat-faced beveled edge tablets debossed with "LCI" over "1353" on one side and "2" on the other side. Available in bottles of 100 (NDC #: 0527-1353-01).
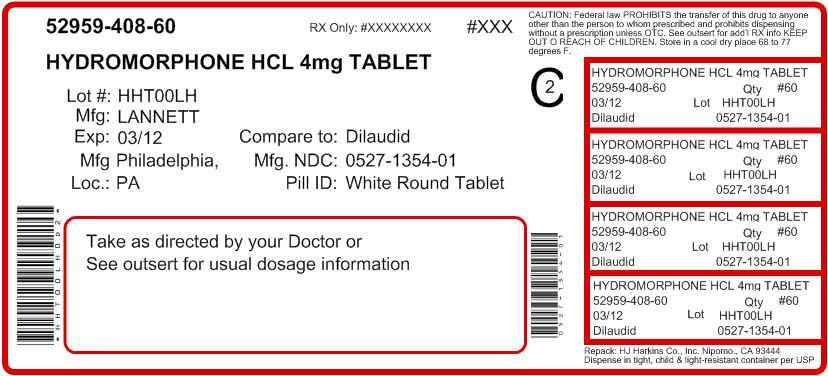
Hydromorphone Hydrochloride Tablets USP, 4 mg are white, round, flat-faced beveled edge tablets debossed with "LCI" over "1354" on one side and "4" on the other side. Available in bottles of 100 (NDC #: 0527-1354-01).
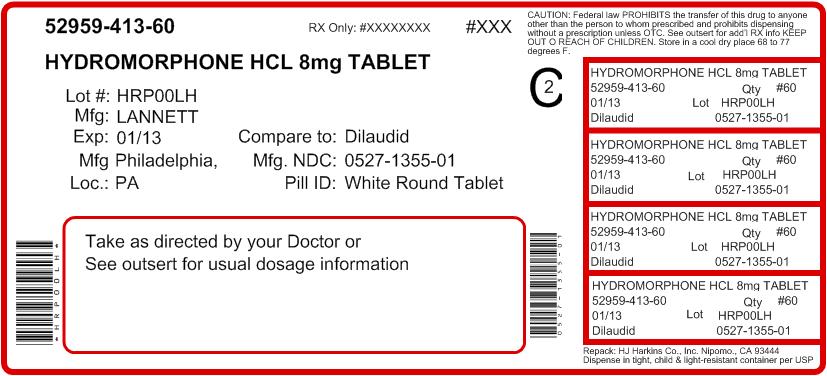
Hydromorphone Hydrochloride Tablets USP, 8 mg are white, round, flat-faced beveled edge, scored, tablets debossed with "LCI" over "1355" on one side and plain on the other side. Available in bottles of 100 (NDC #: 0527-1355-01).
A schedule II Narcotic. DEA Order Form is Required.
Manufactured by:
Lannett Company, Inc.
Philadelphia, PA 19136
Revised October 2006
Repacked by:
H.J. Harkins Company, Inc.
513 Sandydale Drive
Nipomo, CA 93444
PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label
NDC 0527-1353-01
Lannett
HYDROMORPHONE
HYDROCHLORIDE
TABLETS, USP
CII
2 mg
Rx Only
100 TABLETS