DESCRIPTION
hydrochloride(Propranolol is a synthetic beta-adrenergic receptor blocking agent chemically described as 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, hydrochloride,(±)-. It’s molecular and structural formulae are:
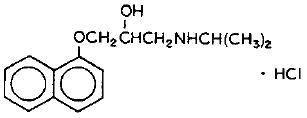
Propranolol hydrochloride is a stable, white, crystalline solid which is readily soluble in water and in ethanol. Its molecular weight is 295.80.
Propranolol hydrochloride tablets, USP are available as 10 mg, 20 mg, 40 mg, 60 mg, and 80 mg tablets for oral administration.
The inactive ingredients contained in propranolol hydrochloride tablets, USP are: lactose monohydrate, corn starch, sodium starch glycolate, magnesium stearate, and povidone. In addition, propranolol hydrochloride tablets, USP 10 mg, 40 mg and 80 mg contain and C yellow No.6 Aluminium Lake and Color D and C Yellow No. 10; propranolol hydrochloride tablets, USP 20 mg and 40mg contain FD and C Blue No.1 and propranolol hydrochloride tablets, USP 60 mg contain D and C Red No. 30 Lake.
CONTRAINDICATIONS
Propranolol is contraindicated in 1) cardiogenic shock; 2) sinus bradycardia and greater than first degree block; 3) bronchial asthma; and 4) in patients with known hypersensitivity to propranolol hydrochloride.
ADVERSE REACTIONS
The following adverse events were observed and have been reported in patients using propranolol.
Cardiovascular: Bradycardia; congestive heart failure; intensification of AV block; hypotension; paresthesia of hands; thrombocytopenic purpura; arterial insufficiency, usually of the Raynaud type.
Central Nervous System: Lightheadedness, mental depression manifested by insomnia, lassitude, weakness, fatigue; catatonia; visual disturbances; hallucinations; vivid dreams; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics. For immediate-release formulations, fatigue, lethargy, and vivid dreams appear dose-related.
Gastrointestinal: Nausea, vomiting, epigastric distress, abdominal cramping, diarrhea, constipation, mesenteric arterial thrombosis, ischemic colitis.
Allergic: Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, pharyngitis and agranulocytosis; erythematous rash, fever combined with aching and sore throat; laryngospasm, and respiratory distress.
Respiratory: Bronchospasm.
Hematologic: Agranulocytosis, nonthrombocytopenic purpura, thrombocytopenic purpura.
Autoimmune: Systemic lupus erythematosus (SLE).
Skin and mucous membranes: Stevens-Johnson Syndrome, toxic epidermal necrolysis, dry eyes, exfoliative dermatitis, erythema multiforme, urticaria, alopecia, SLE-like reactions, and psoriasiform rashes. Oculomucocutaneous syndrome involving the skin, serous membranes and conjunctivae reported for a beta-blocker (practolol) have not been associated with propranolol.
Genitourinary: Male impotence; Peyronie’s disease.
OVERDOSAGE
Propranolol is not significantly dialyzable. In the event of overdosage or exaggerated response, the following measures should be employed:
General: If ingestion is or may have been recent, evacuate gastric contents, taking care to prevent pulmonary aspiration.
Supportive Therapy: Hypotension and bradycardia have been reported following propranolol overdose and should be treated appropriately. Glucagon can exert potent inotropic and chronotropic effects and may be particularly useful for the treatment of hypotension or depressed myocardial function after a propranolol overdose. Glucagon should be administered as 50-150 mcg/kg intravenously followed by continuous drip of 1-5 mg/hour for positive chronotropic effect. Isoproterenol, dopamine or phosphodiesterase inhibitors may also be useful. Epinephrine, however, may provoke uncontrolled hypertension. Bradycardia can be treated with atropine or isoproterenol. Serious bradycardia may require temporary cardiac pacing.
The electrocardiogram, pulse, blood pressure, neurobehavioral status and intake and output balance must be monitored. Isoproterenol and aminophylline may be used for bronchospasm.
HOW SUPPLIED
Propranolol hydrochloride tablets, USP are supplied as follows:
10mg: Each tablet is an orange colored, round, biconvex tablet, embossed with "P" and "10" on either side of the breakline on one side and plain on the other side.
Each 10 mg tablet contains 10 mg propranolol hydrochloride USP and is supplied in the following packages sizes:
Bottles of 30 tablets NDC 23155-110-03
Bottles of 60 tablets NDC 23155-110-06
Bottles of 100 tablets NDC 23155-110-01
Bottles of 500 tablets NDC 23155-110-05
Bottles of 1000 tablets NDC 23155-110-10
20mg: Each tablet is a blue colored, round, biconvex tablet, embossed with "P" and "20" on either side of the breakline on one side and plain on the other side.
Each 20 mg tablet contains 20 mg of propranolol hydrochloride USP and is supplied in the following package sizes:
Bottles of 30 tablets NDC 23155-111-03
Bottles of 60 tablets NDC 23155-111-06
Bottles of 100 tablets NDC 23155-111-01
Bottles of 500 tablets NDC 23155-111-05
Bottles of 1000 tablets NDC 23155-111-10
40mg: Each tablet is a green colored, round, biconvex tablet, embossed with "P" and "40"on either side of the breakline on one side and plain on the other side.
Each 40 mg tablet contains 40 mg of propranolol hydrochloride USP and is supplied in the following package sizes:
Bottles of 30 tablets NDC 23155-112-03
Bottles of 60 tablets NDC 23155-112-06
Bottles of 100 tablets NDC 23155-112-01
Bottles of 500 tablets NDC 23155-112-05
Bottles of 1000 tablets NDC 23155-112-10
60mg: Each tablet is a pink colored, round, biconvex tablet, embossed with "P" and "60" on either side of the breakline on one side and plain on the other side.
Each 60 mg tablet contains 60 mg of propranolol hydrochloride USP and is supplied in the following package sizes:
Bottles of 30 tablets NDC 23155-113-03
Bottles of 60 tablets NDC 23155-113-06
Bottles of 100 tablets NDC 23155-113-01
Bottles of 500 tablets NDC 23155-113-05
Bottles of 1000 tablets NDC 23155-113-10
80mg: Each tablet is a yellow colored, round, biconvex tablet, embossed with "P" and "80" on either side of the breakline on one side and plain on the other side.
Each 80 mg tablet contains 80 mg of propranolol hydrochloride USP and is supplied in the following package sizes:
Bottles of 30 tablets NDC 23155-114-03
Bottles of 60 tablets NDC 23155-114-06
Bottles of 100 tablets NDC 23155-114-01
Bottles of 500 tablets NDC 23155-114-05
Bottles of 1000 tablets NDC 23155-114-10
Storage: Store at 20°-25°C (68-(°77(°F); excursions permitted to 15°-30(°C (59(°-86(°F) [see USP Controlled Room Temperature]
Protect from light.
Dispense in a well closed, light-resistant container as defined in USP.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.